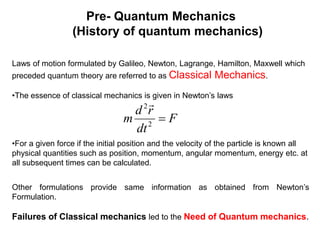
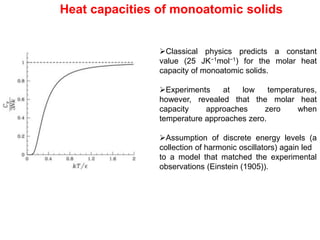
- Classical mechanics fails to fully describe phenomena at the atomic scale such as blackbody radiation, heat capacities of solids at low temperatures, and the photoelectric effect. This led to the development of quantum mechanics.
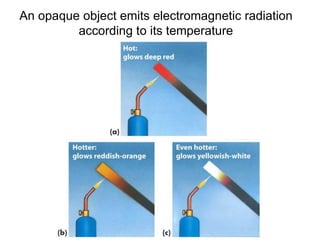
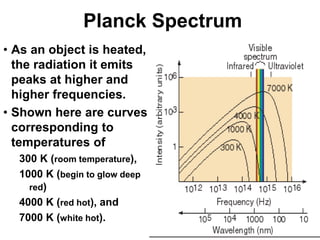
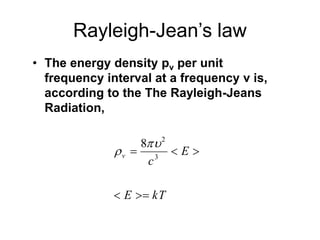
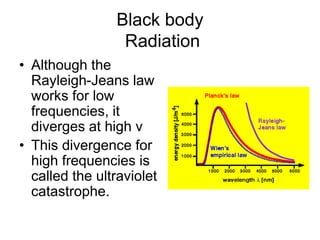
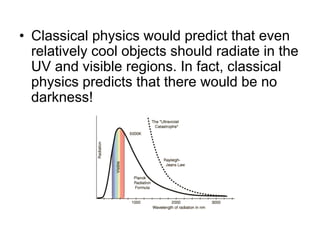
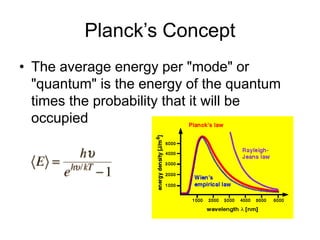
- Planck explained blackbody radiation by proposing that electromagnetic radiation is emitted and absorbed in discrete 'quanta' with energy proportional to frequency. This ended the "ultraviolet catastrophe" of classical physics.
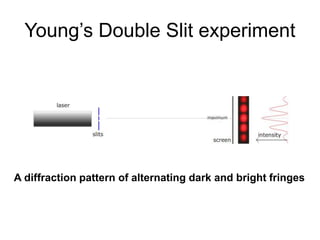
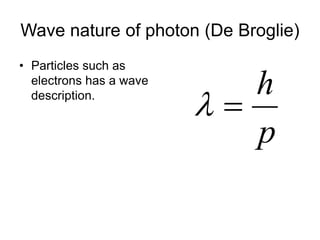
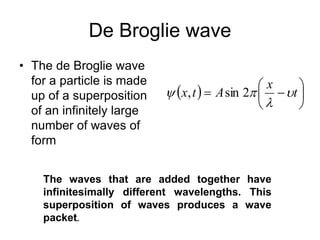
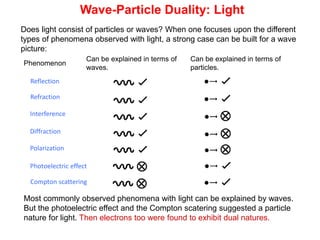
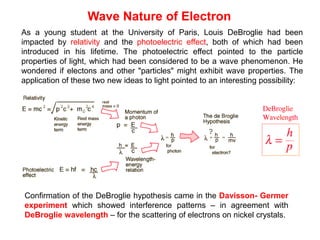
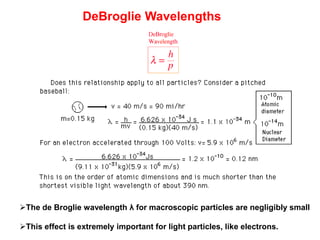
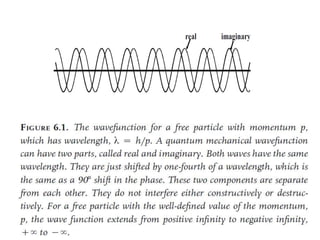
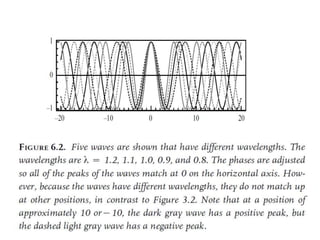
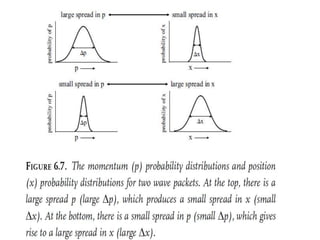
- De Broglie associated waves with all particles, suggesting light has both wave-like and particle-like properties. This was confirmed through experiments and led to the modern concept of wave-particle duality.















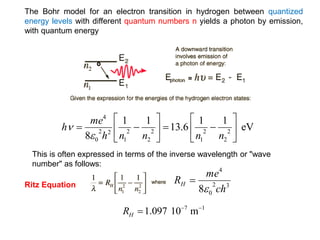
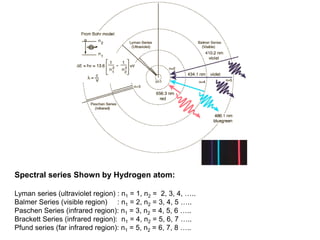

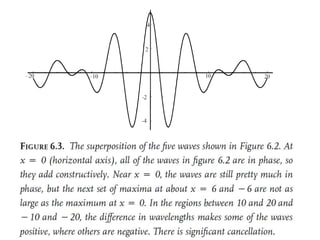
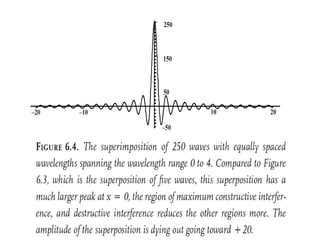
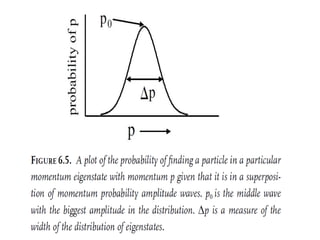
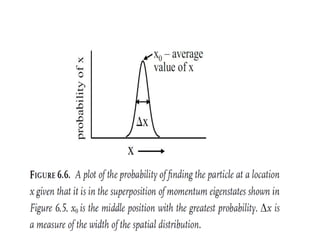


![Wave equation for the harmonic motion
)
(
)
(
)
(
8
]
2
[
]
2
[
2
2
1
)
(
)
(
4
)
(
2
)
(
2
2
2
2
2
1
2
1
2
2
2
2
2
2
2
2
2
x
V
E
dx
x
d
m
h
V
E
m
h
p
h
V
E
m
p
V
m
p
V
mv
E
x
dx
x
d
x
dx
x
d
](https://image.slidesharecdn.com/quantumlecturecml1-221102131032-3e406e93/85/Quantum_lecture_CML_1-ppt-52-320.jpg)