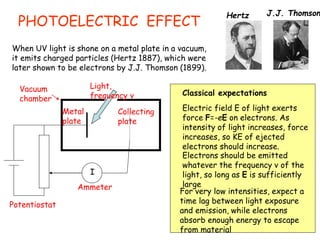
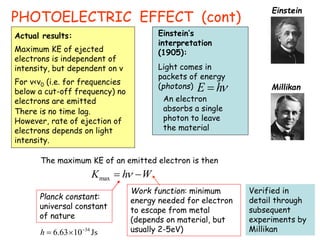
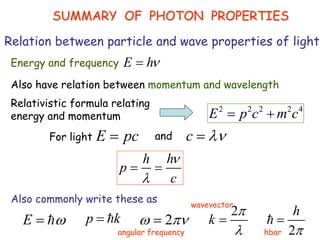
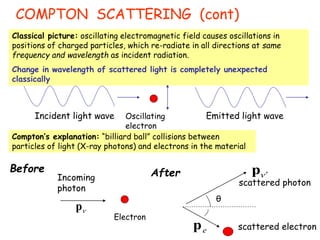
Wave-particle duality is demonstrated through several experiments:
1) The photoelectric effect shows that light behaves as particles (photons) that transfer discrete packets of energy.
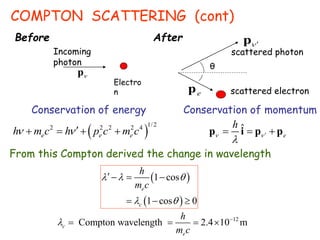
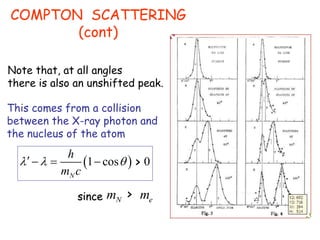
2) Compton scattering shows that X-rays behave as particles that can collide with and transfer momentum to electrons.
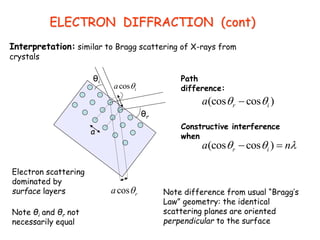
3) Electron diffraction demonstrates the wave-like properties of electrons through interference and diffraction patterns.
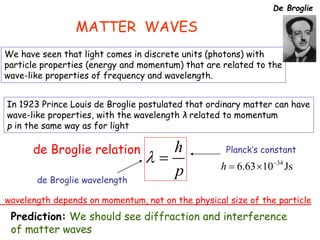
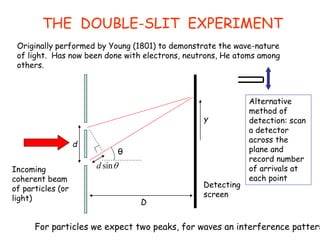
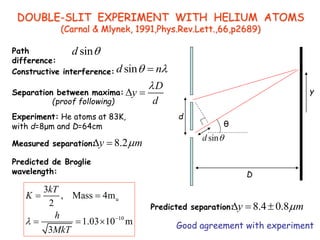
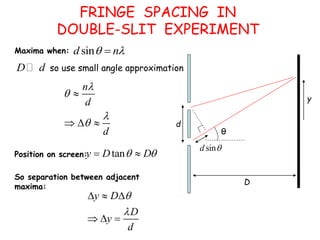
4) The double-slit experiment shows interference patterns for particles like electrons, atoms, and molecules, demonstrating their wave-like properties.
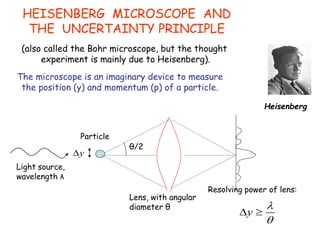
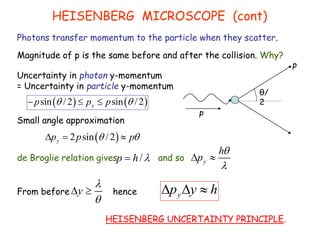
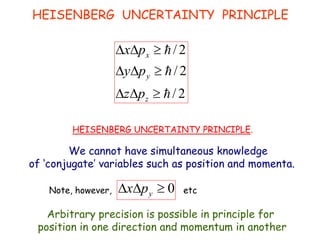
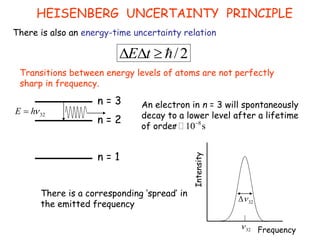
Heisenberg's uncertainty principle mathematically quantifies the wave-particle duality - the more precisely one property of a particle is measured, the less precisely its