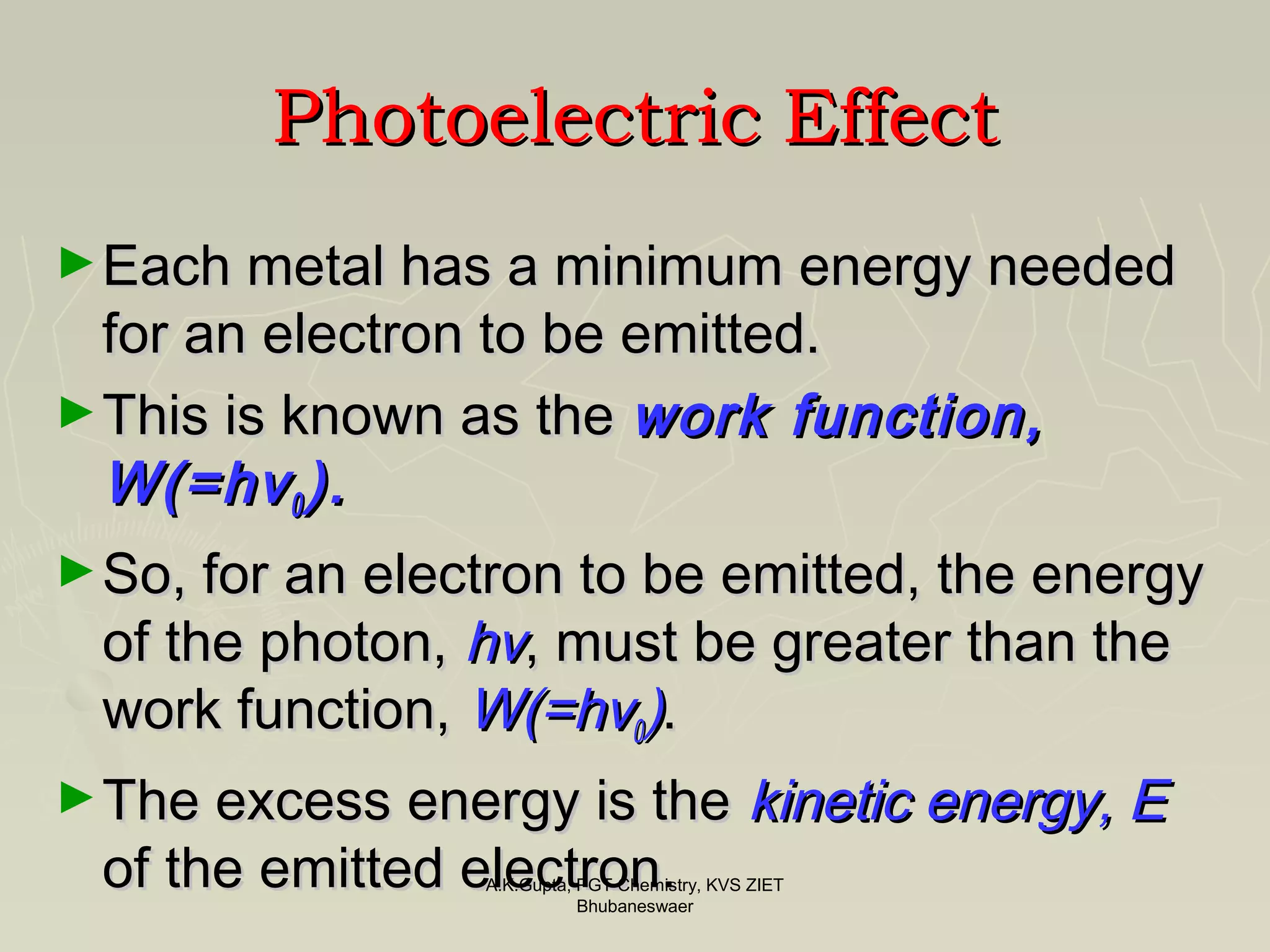
The document discusses the photoelectric effect and how it helped lead to Einstein's fame. It describes experiments showing that shining blue light on a metal foil causes electrons to be emitted, while red light does not. Increasing the intensity of red light also does not cause emission. Einstein explained these results by proposing that light consists of discrete quanta of energy, with higher frequency light having more energy per quantum. His theory that the energy of emitted electrons depends on the frequency, not intensity, of light helped establish the quantum nature of light.