This document provides an overview of basic chemistry concepts including:
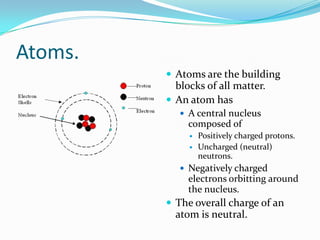
- Atoms are made of protons, neutrons, and electrons. Ions are formed when atoms gain or lose electrons.
- Elements are substances made of only one type of atom, with carbon, hydrogen, oxygen, and nitrogen being the most important elements in living things.
- Molecules are formed from chemical bonds between atoms, and compounds contain two or more different types of atoms bonded together. Covalent and ionic bonding allow atoms to combine via electron sharing or transfer.





![Chemical bonding.
The atoms that make up a molecule are held together
by either
Covalent bonds
Ionic bonds, or
Metallic bonding (not relevant here).
Atoms combine with other atoms in order to become
more stable.
Atoms are most stable when they have a full outer shell
[like the noble gases in Gp18].](https://image.slidesharecdn.com/bi033-02basicchemistry-2012-120310192635-phpapp02/85/Basic-chemistry-7-320.jpg)




