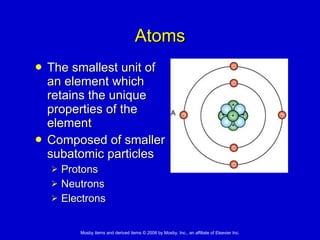
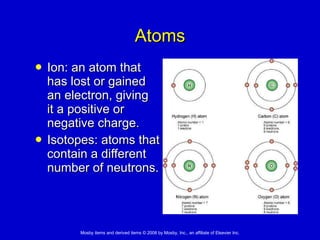
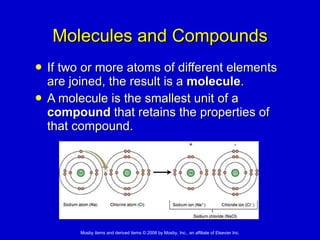
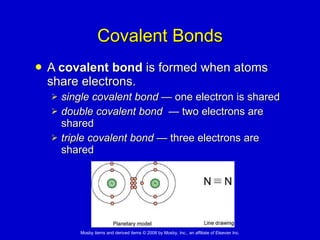
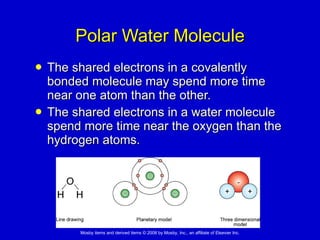
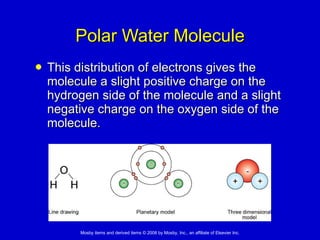
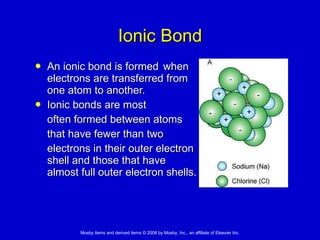
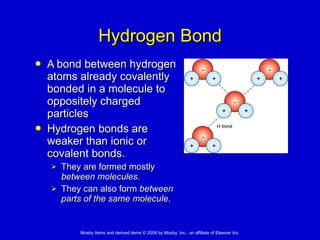
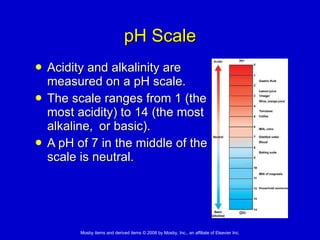
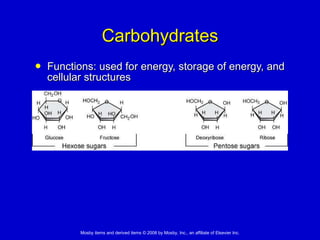
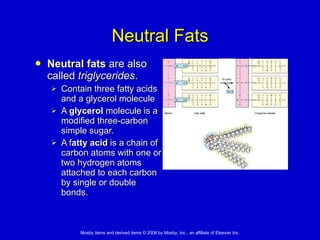
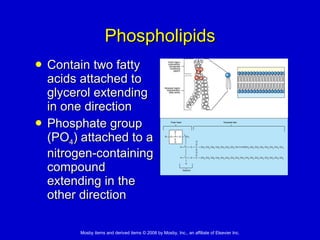
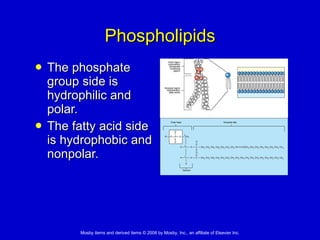
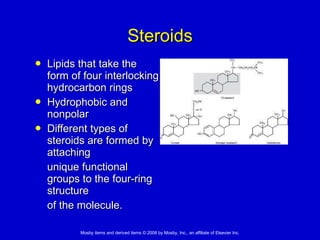
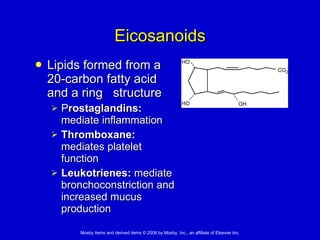
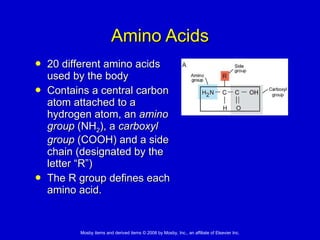
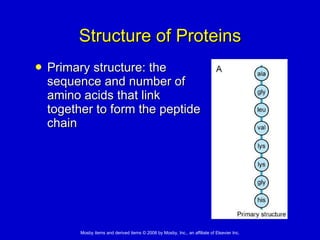
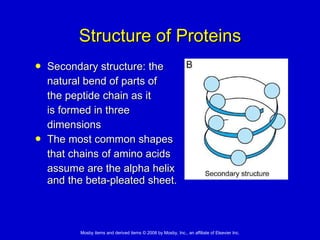
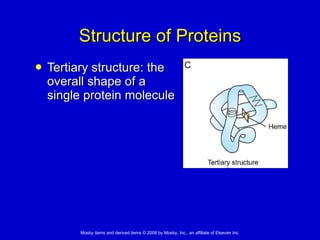
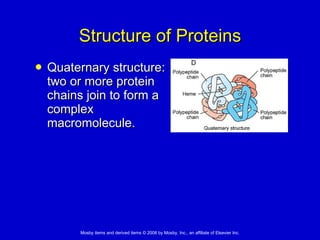
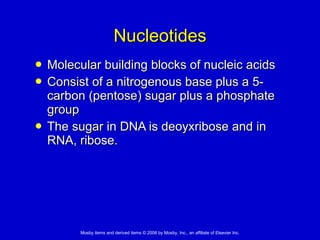
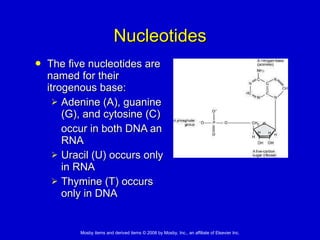
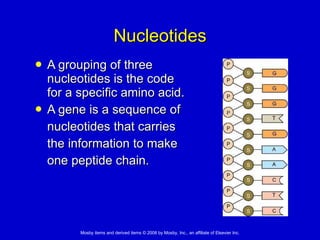
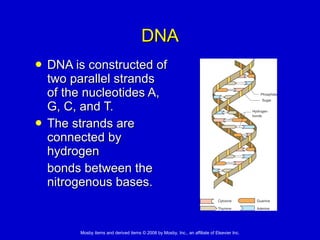
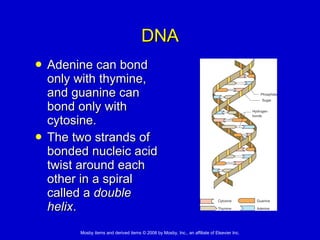
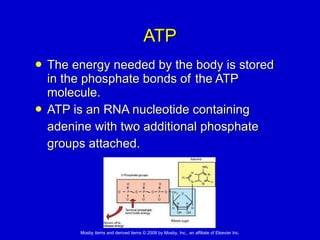
This document summarizes key concepts in chemistry that are relevant to understanding the chemical basis of life. It defines matter and its composition of elements and atoms. It describes the structure of atoms including protons, neutrons, electrons and electron shells. It explains the formation of molecules, compounds, and different types of chemical bonds. It discusses the unique properties of water and its role in biological systems. It also summarizes the main macromolecules that make up living things - carbohydrates, lipids, proteins and nucleic acids - and describes their structure and functions.