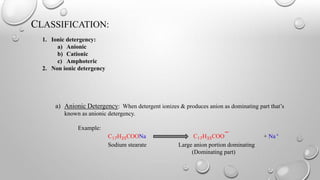
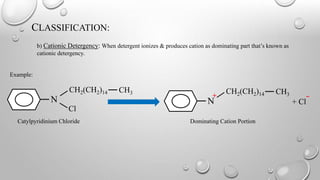
Detergency is the process of removing dirt and oil from fabric surfaces using surfactants or detergents. The detergent works by reducing the surface tension between the fabric and dirt/oil, allowing the dirt/oil to be converted from a horizontal to spherical shape and more easily removed. Detergent molecules form spherical micelle structures with their hydrophobic tails inward and hydrophilic heads outward. Dirt and oil particles are encapsulated inside the micelle structures and removed from the fabric surface. Detergents are classified as ionic (anionic, cationic, amphoteric) or non-ionic depending on whether they ionize in solution.






![ MECHANISM:
ɵ
Ts TLS
TL
At the adhering situation of dirt, its actual shape will
be detached by the interaction of the following
forces:
i. TL = Surface tension between oil and surrounding.
ii. TS = Surface tension between fibre and water.
iii. TLS = Surface tension between fibre and oil.
iv. ɵ = Contact angel.
From Young’s equation we get,
TS = TLS + TL cosƟ
cosƟ =
TS−TLS
TL
TL = TS - TLS [When Ɵ=0, cosƟ= 1]
TS = TLS + TL
TLS = TS – TL](https://image.slidesharecdn.com/detergencyforpublish-181109073702/85/Detergency-7-320.jpg)