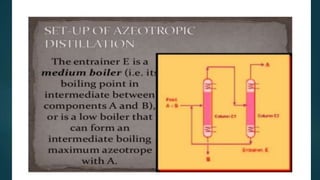
Distillation is a physical separation process that separates mixtures based on differences in boiling points. There are several types of distillation including simple distillation, fractional distillation, steam distillation, and vacuum distillation. Simple distillation is used when components differ in boiling point by at least 70°C while fractional distillation uses a fractionating column to separate components that have similar boiling points within 25°C. Steam distillation allows purification of heat sensitive compounds by boiling them at a lower temperature using steam.