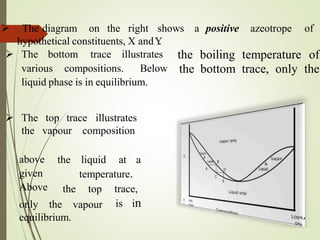
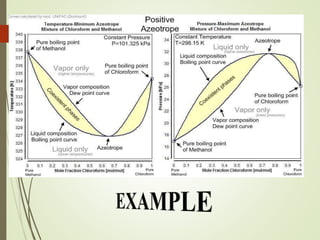
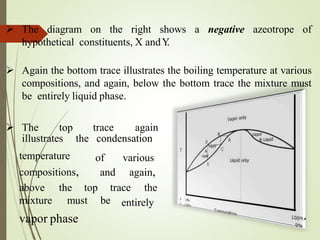
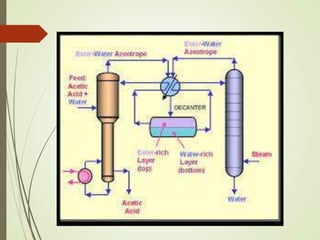
Azeotropic distillation is a distillation technique used to separate mixtures that form a constant-boiling azeotrope. An azeotrope is a mixture of two or more liquids that cannot be separated by simple distillation because the vapor produced has the same composition as the liquid mixture. Entrainers are added to break the azeotrope by changing the volatility of the components. Common applications include removing water from ethanol using cyclohexane as the entrainer. Azeotropic distillation units consist of containers, decanters, and steamers to separate components into different boiling point fractions.