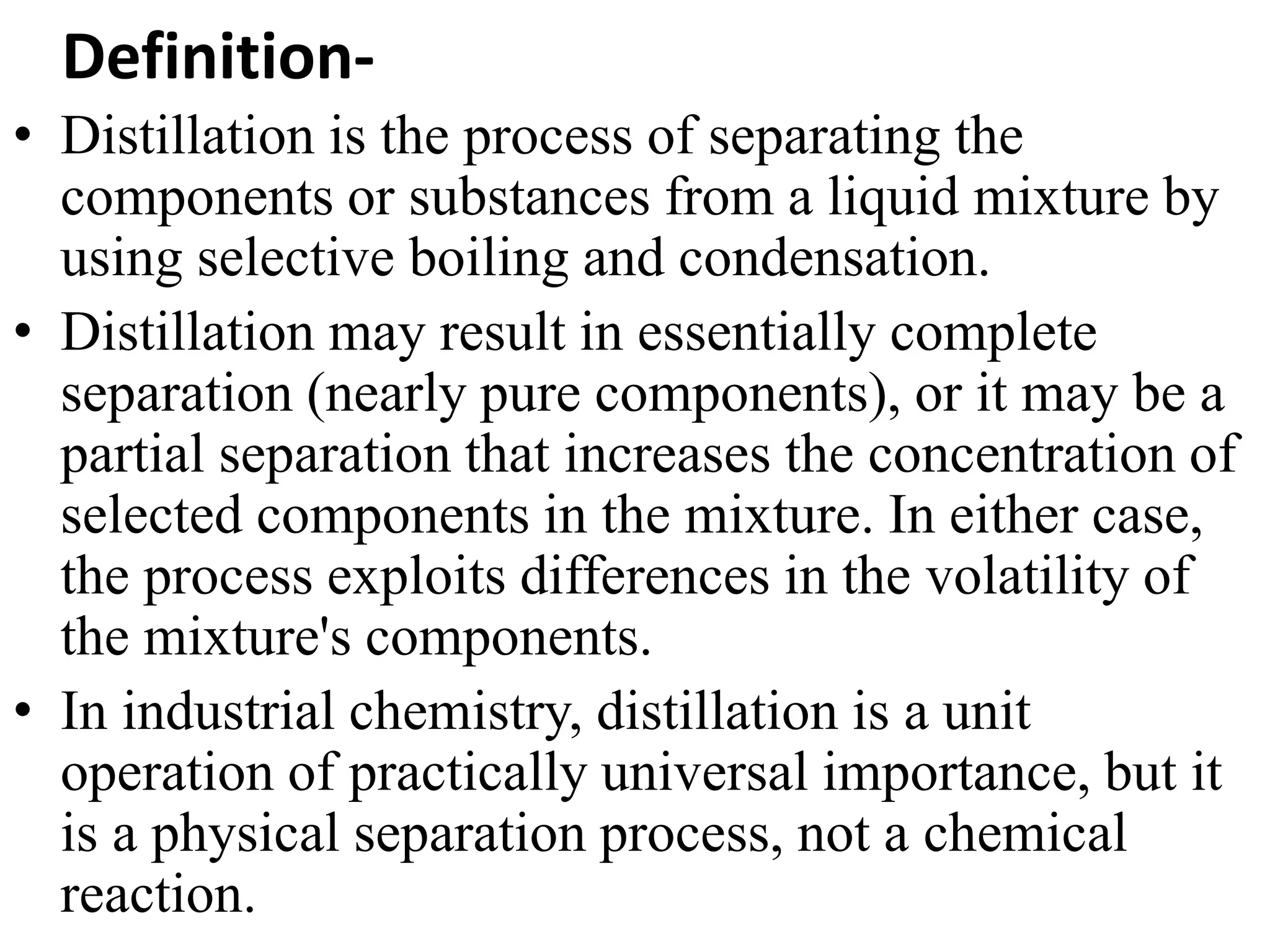
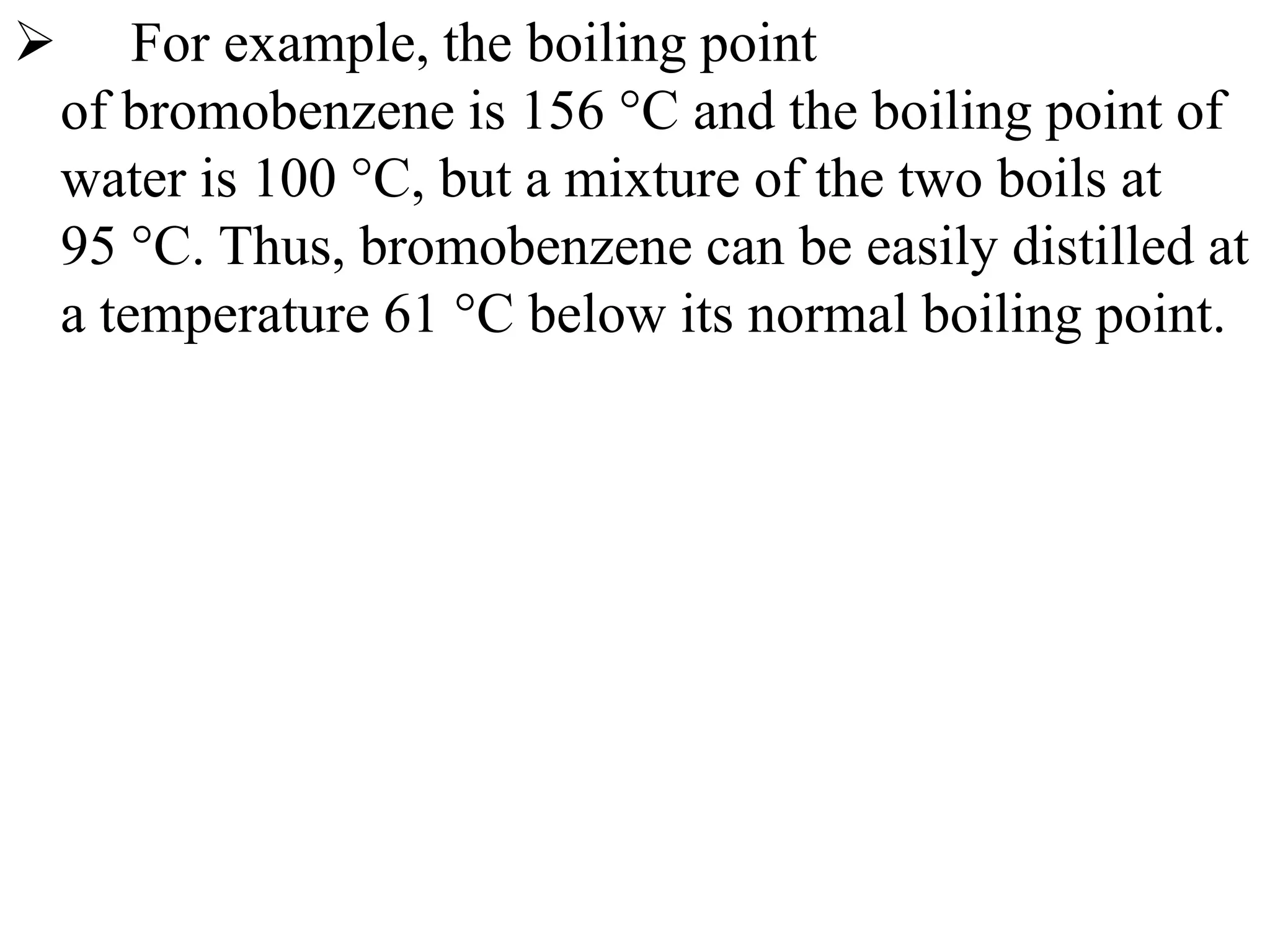
Steam distillation and azeotropic distillation are separation processes.
Steam distillation uses steam to distill temperature sensitive compounds like essential oils at lower temperatures to prevent decomposition. It produces a mixture of water and organic distillate that can be separated.
Azeotropic distillation uses an entrainer added to the original mixture to form a new azeotrope that distills off, allowing separation of the original components which could not be separated otherwise due to forming an azeotrope. Both processes exploit differences in volatility between mixture components to achieve separation.