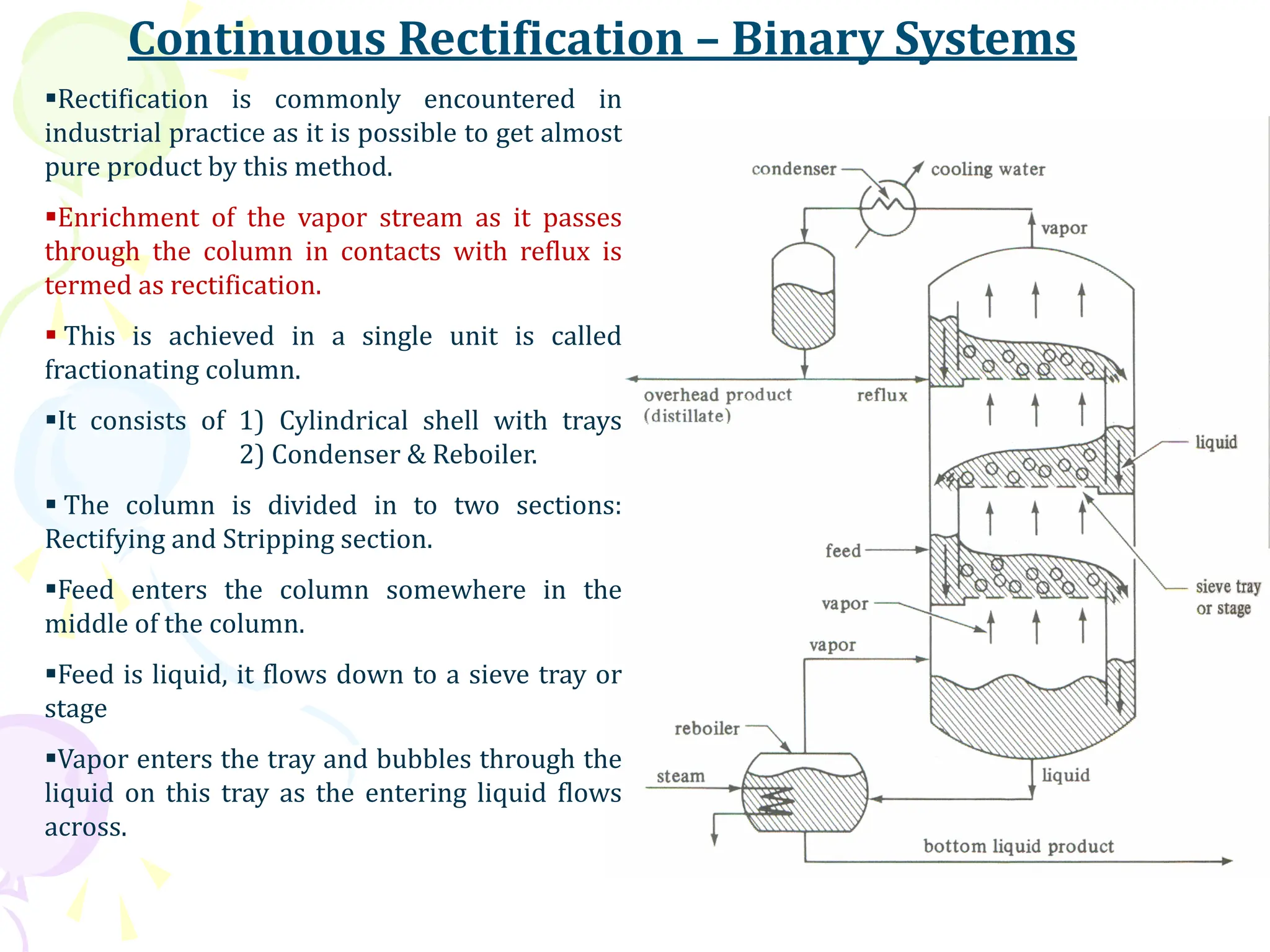
The document provides an in-depth overview of distillation, highlighting its principles, types, and essential theories related to vapor-liquid equilibrium. It discusses the processes of vaporization and condensation, the role of boiling point diagrams, and the significance of azeotropes in separation processes. Additionally, it covers methods such as differential distillation, flash vaporization, and continuous rectification while explaining the McCabe-Thiele method for determining theoretical trays in distillation columns.