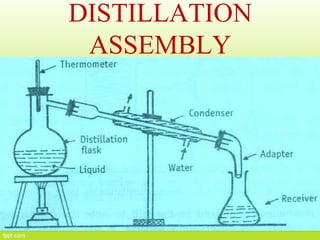
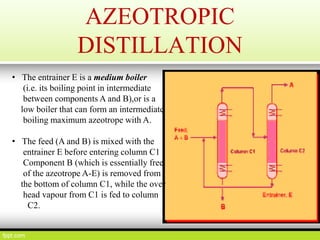
This document discusses distillation, including definitions, applications, Raoult's law, and different types of distillation processes. It describes steam distillation and its setup for separating immiscible liquids like water and essential oils. Azeotropic distillation is explained where an entrainer is added to break or form an azeotrope allowing better separation. Various industrial and medical applications are presented along with diagrams of distillation equipment.