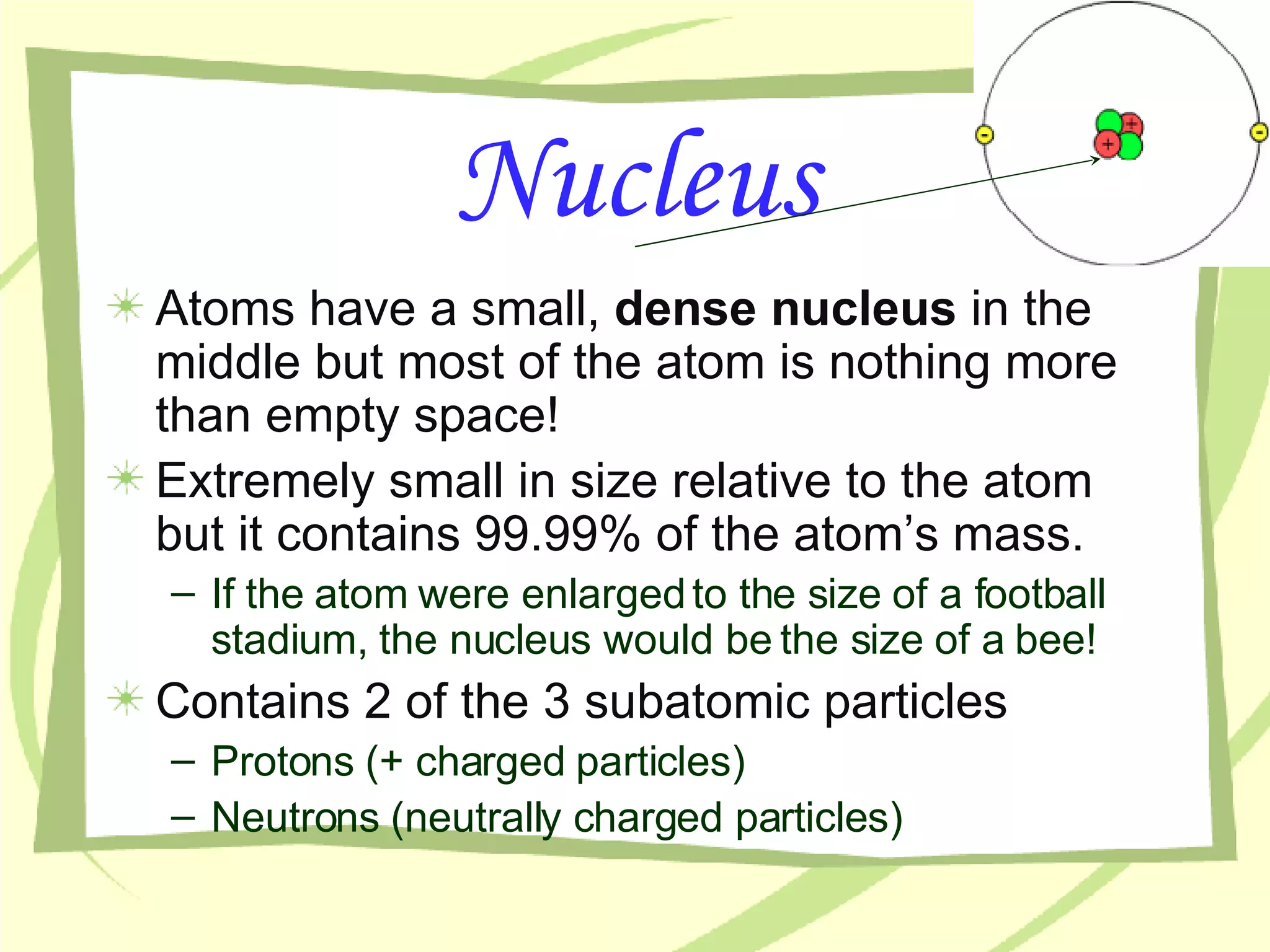
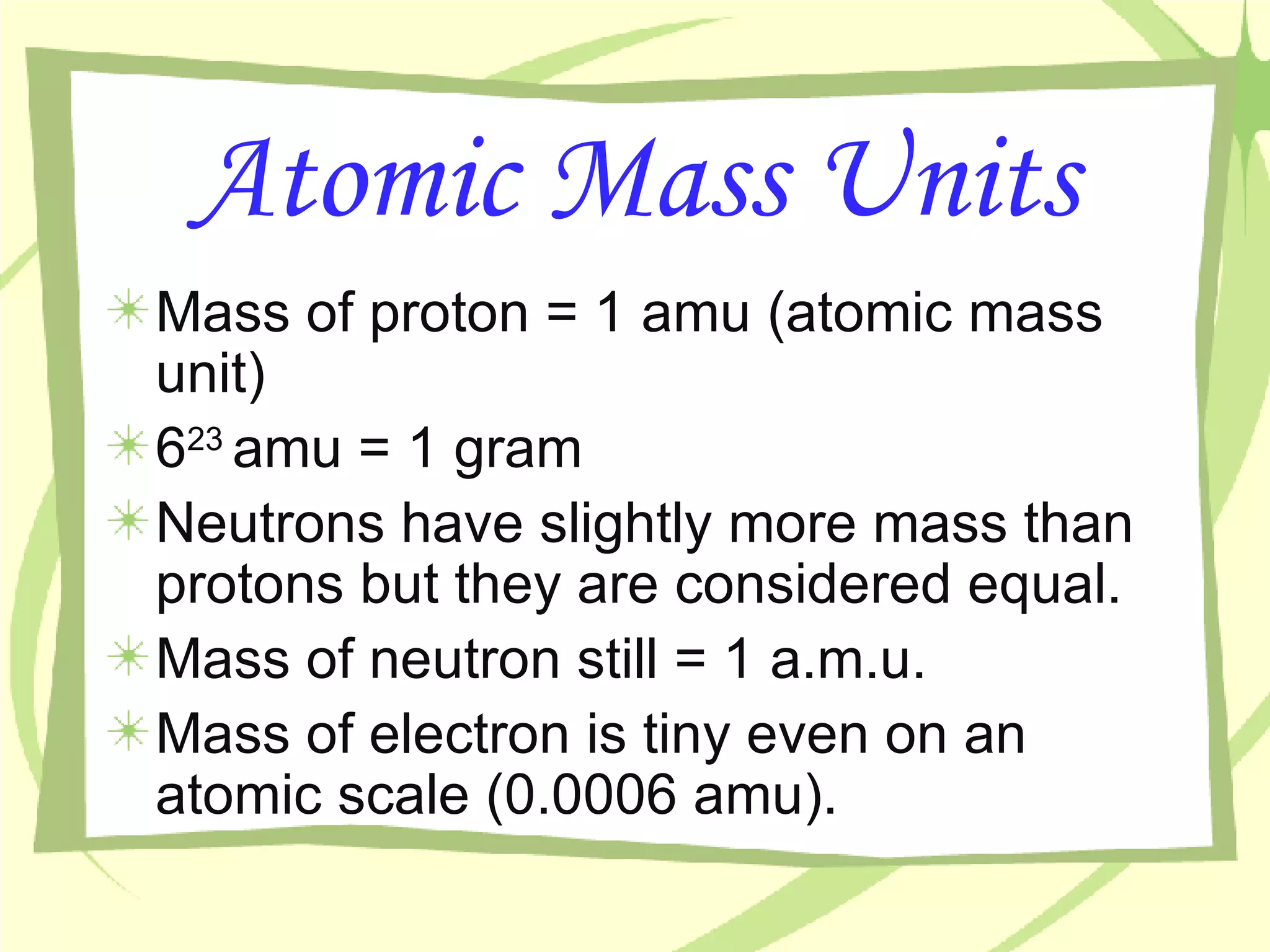
- Atoms are the building blocks of matter and are made up of protons, neutrons, and electrons. The nucleus contains protons and neutrons and accounts for nearly all an atom's mass, while electrons orbit the nucleus.
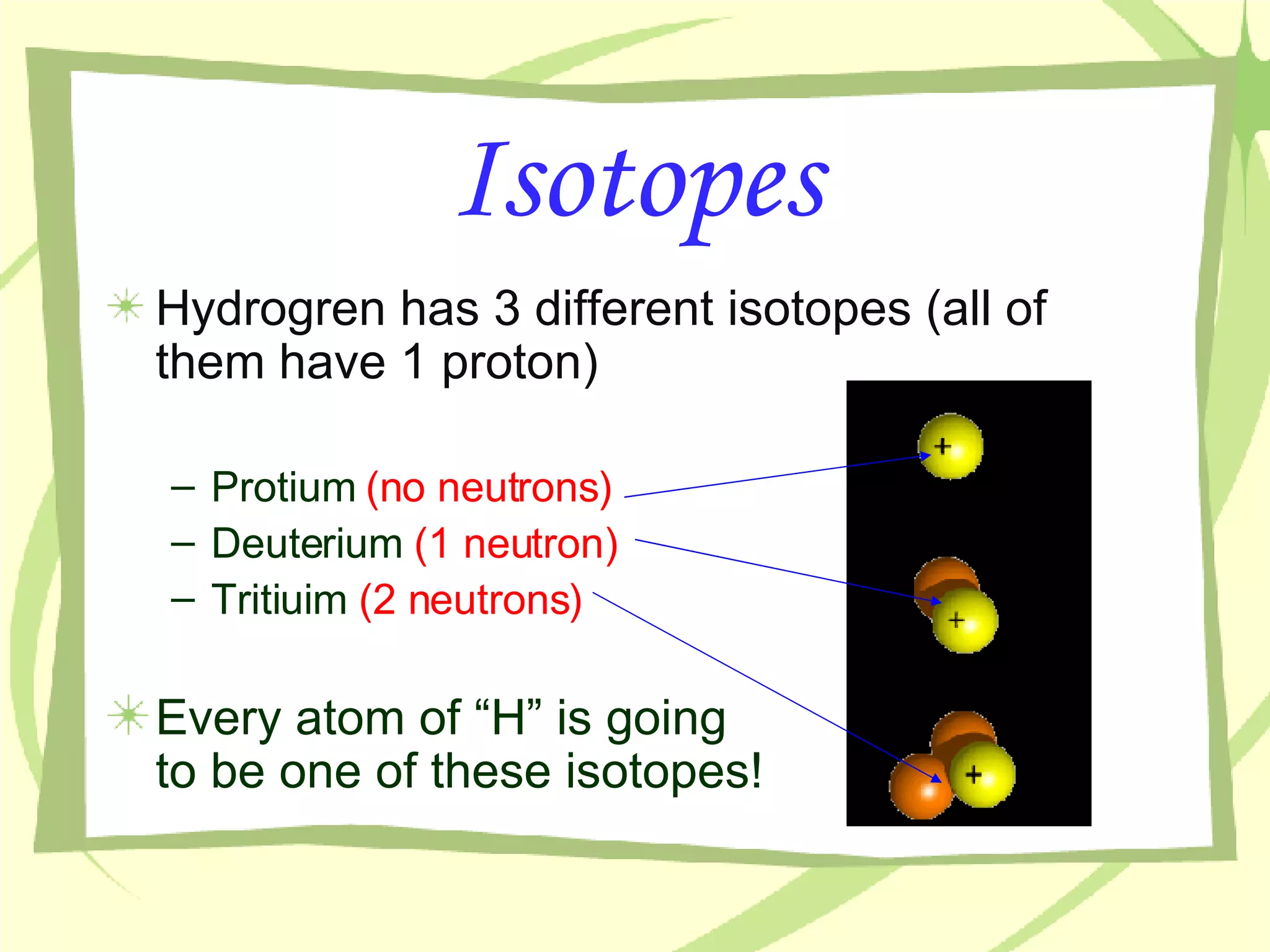
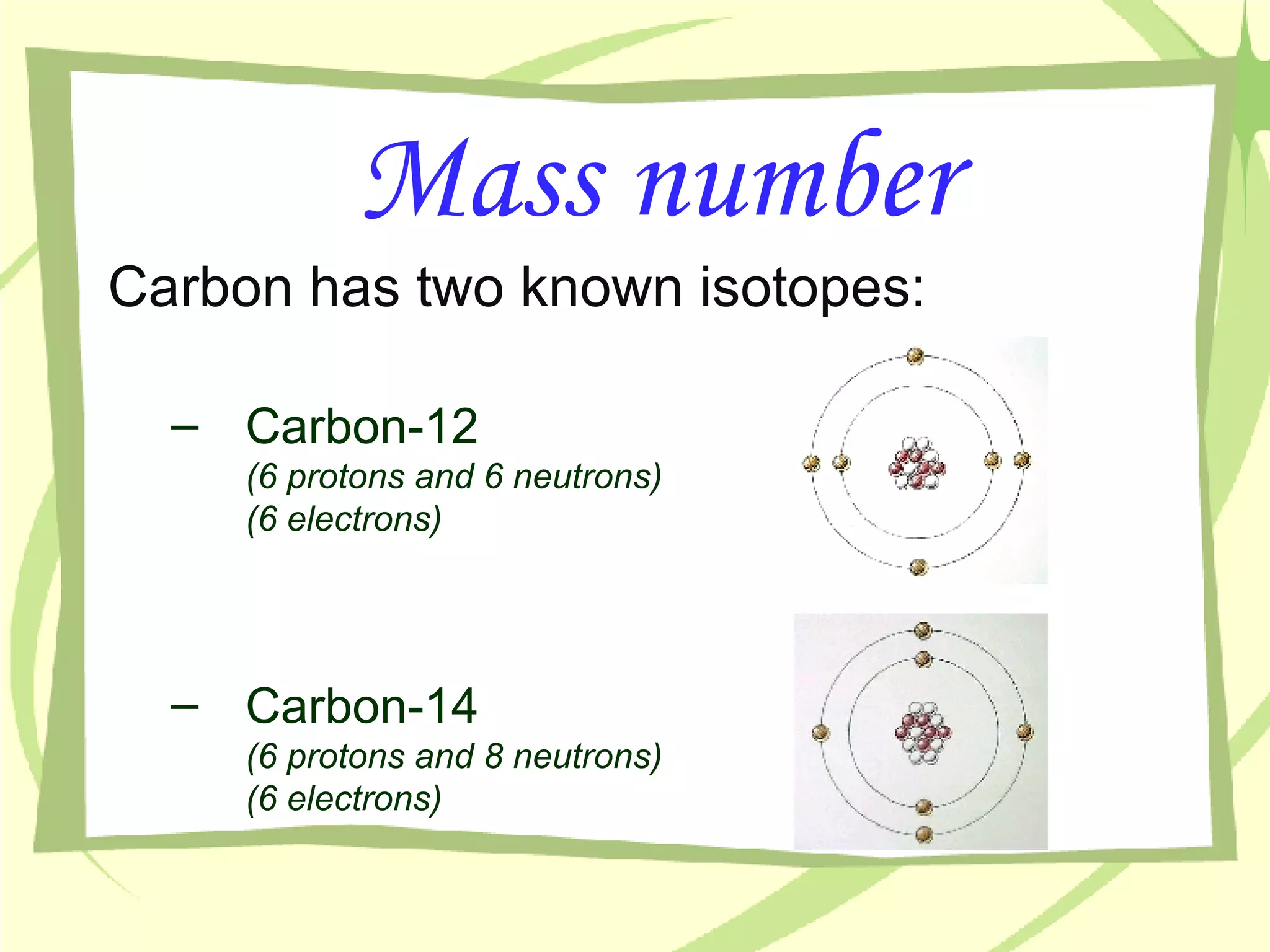
- The number of protons determines the element and cannot be changed. Neutrons can vary between atoms of the same element, creating isotopes of that element.
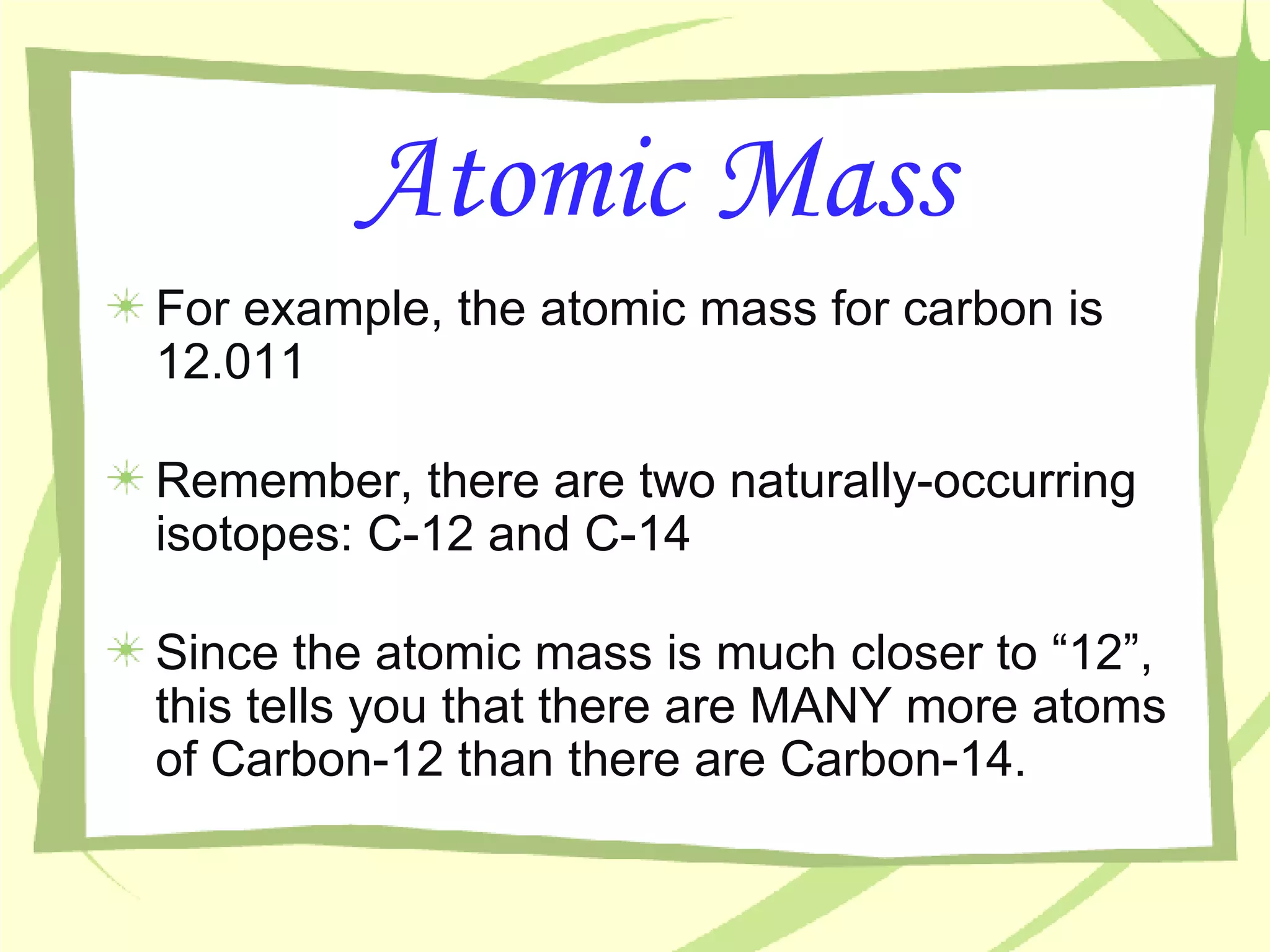
- An atom's mass number is the total number of protons and neutrons, while its atomic mass refers to the average mass of all isotopes of that element as found in nature.