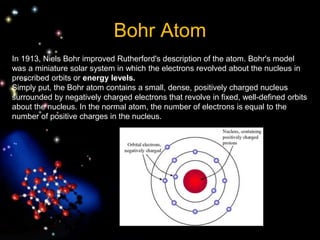
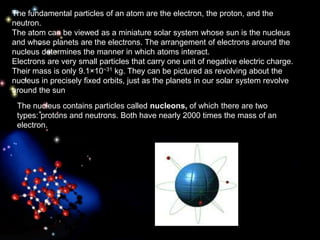
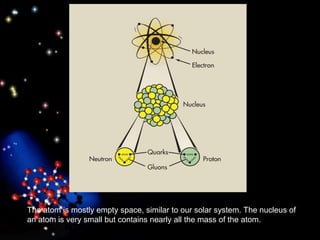
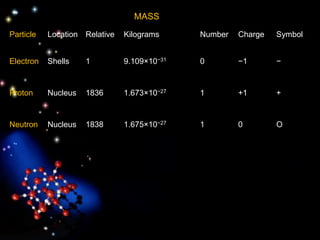
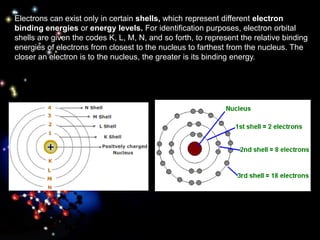
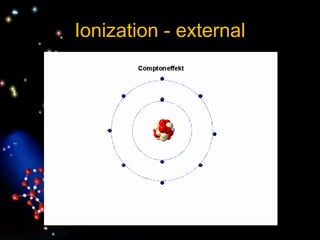
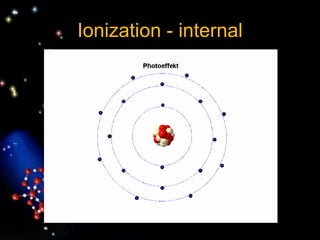
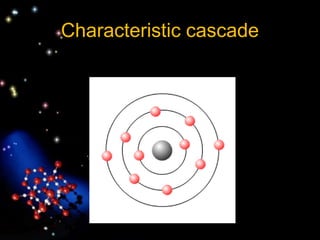
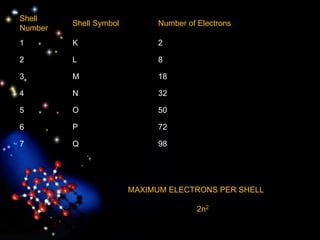
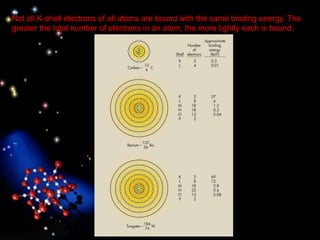
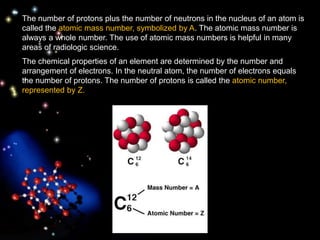
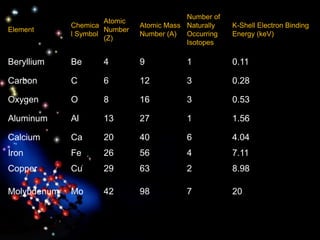
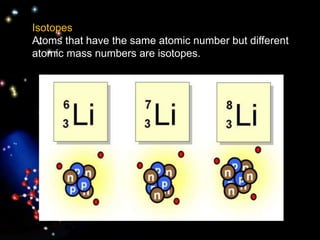
The document discusses the evolution of atomic theories from ancient Greek ideas to modern atomic structure. It covers the Greek concept of atoms as indivisible particles, Dalton's postulation that atoms are basic units that combine to form compounds, Thomson's "plum pudding" model depicting electrons in an atom, and Bohr's model of electrons orbiting the nucleus in fixed shells like planets around the sun. The modern atomic model includes protons and neutrons in the nucleus surrounded by electrons in shells, with the number of protons determining the element.

![The Greeks used the term atom, meaning “indivisible”
[a (not) + temon (cut)] to describe the smallest part of
the four substances of matter.
Greek Atom
The earliest recorded reference to this investigation
comes from the Greeks, several hundred years bc.
Scientists at that time thought that all matter was
composed of four substances: earth, water, air, and fire](https://image.slidesharecdn.com/7248247-221011131756-dc539fc1/85/7248247-ppt-3-320.jpg)