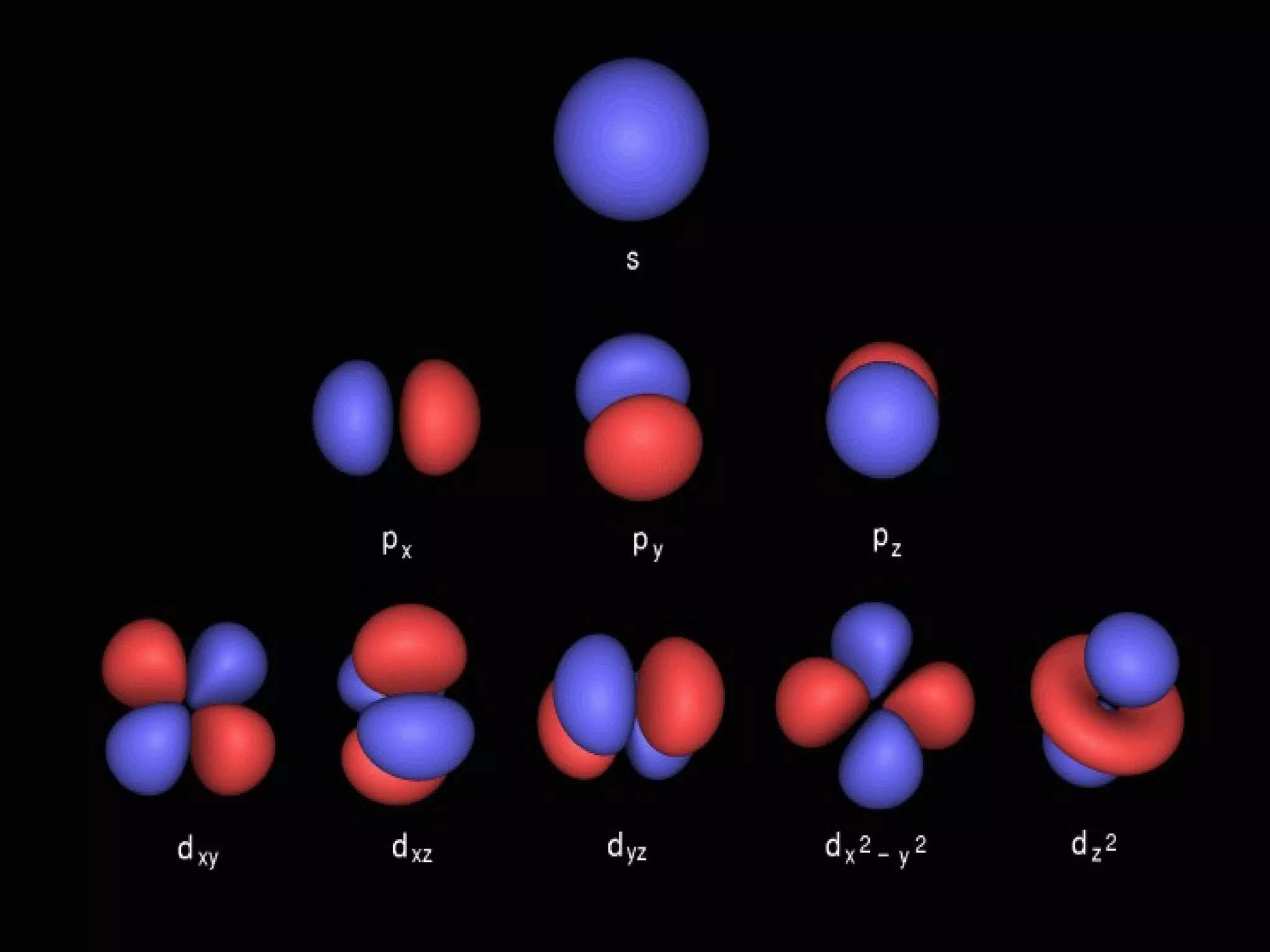
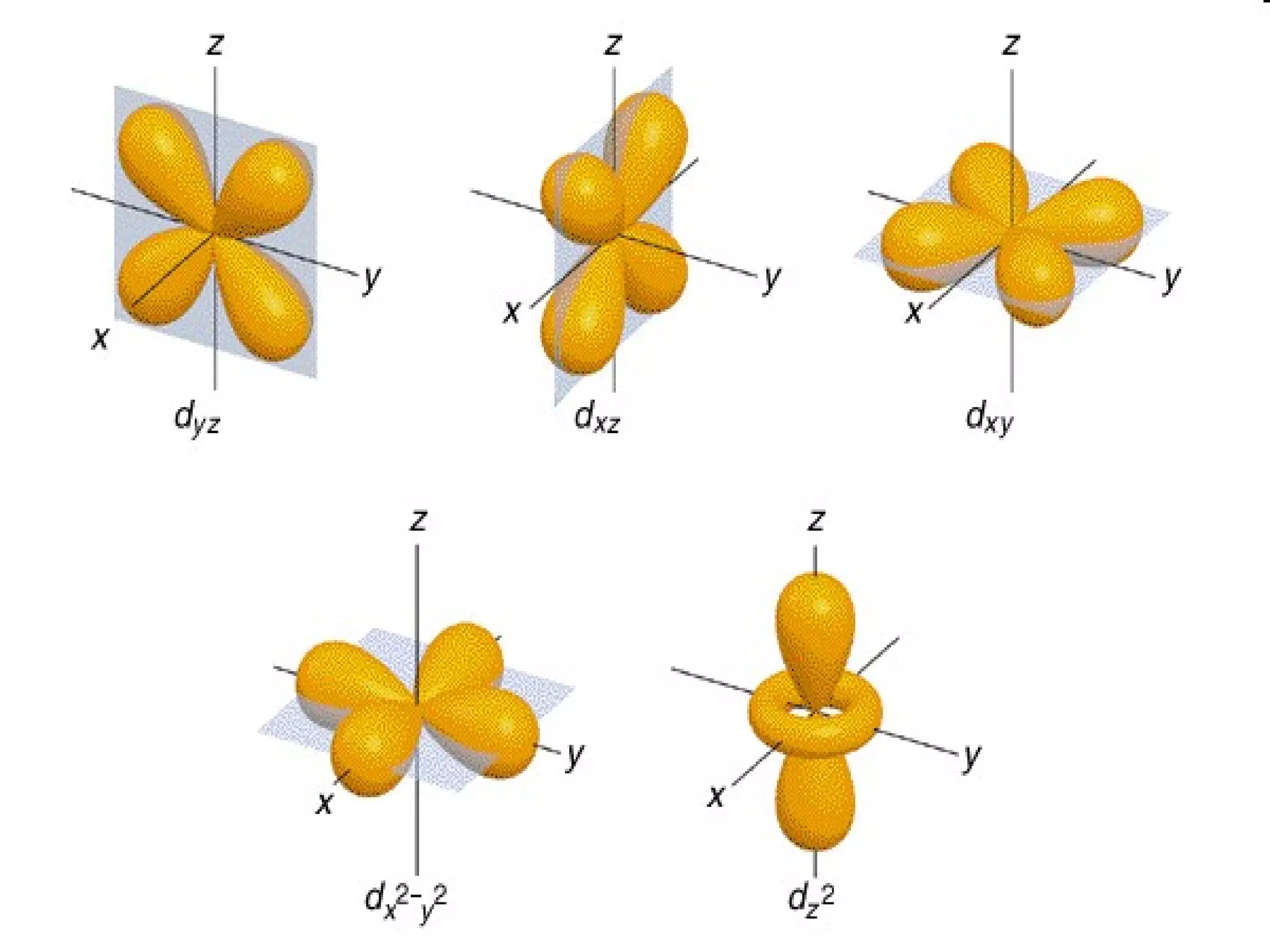
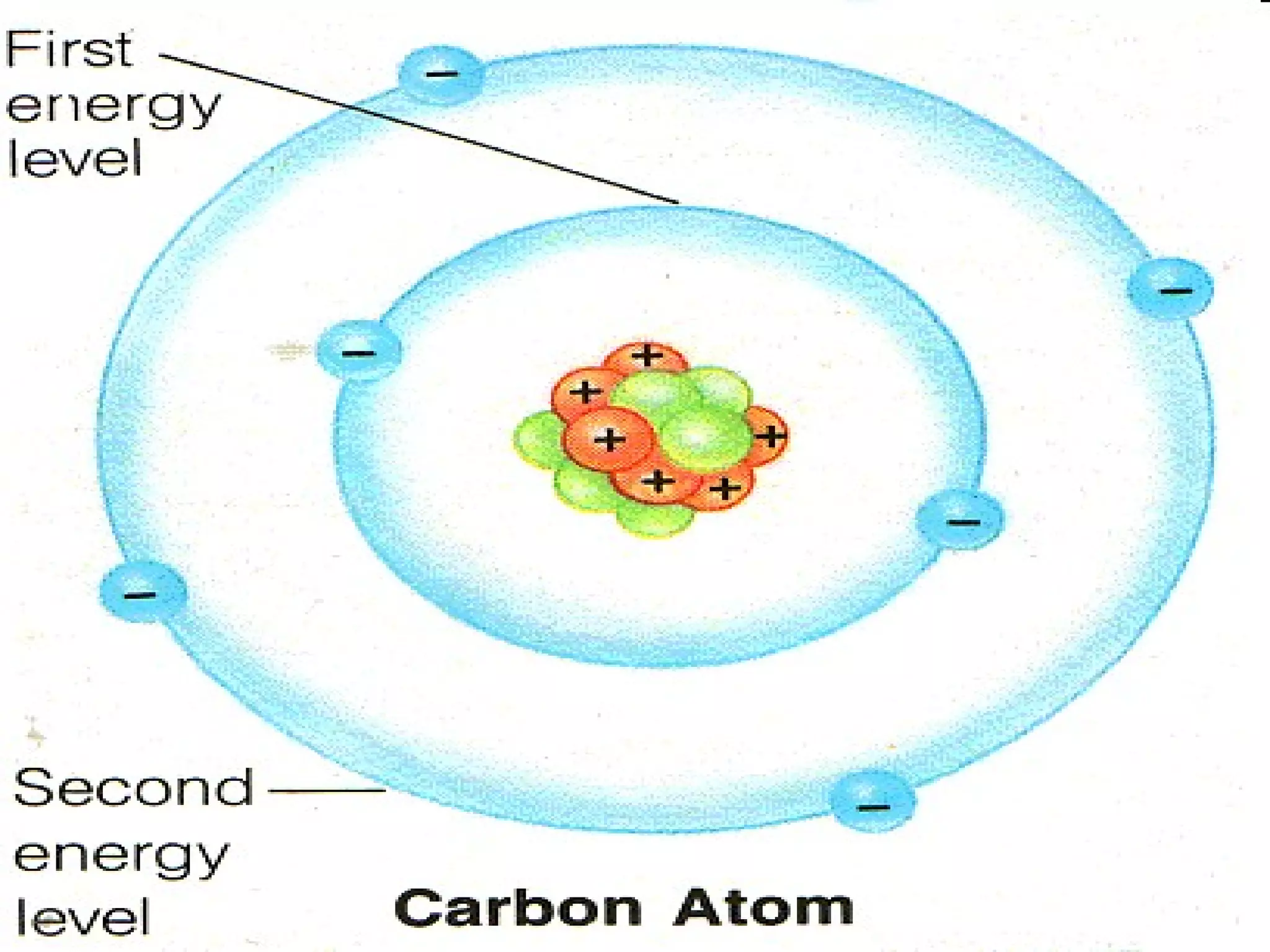
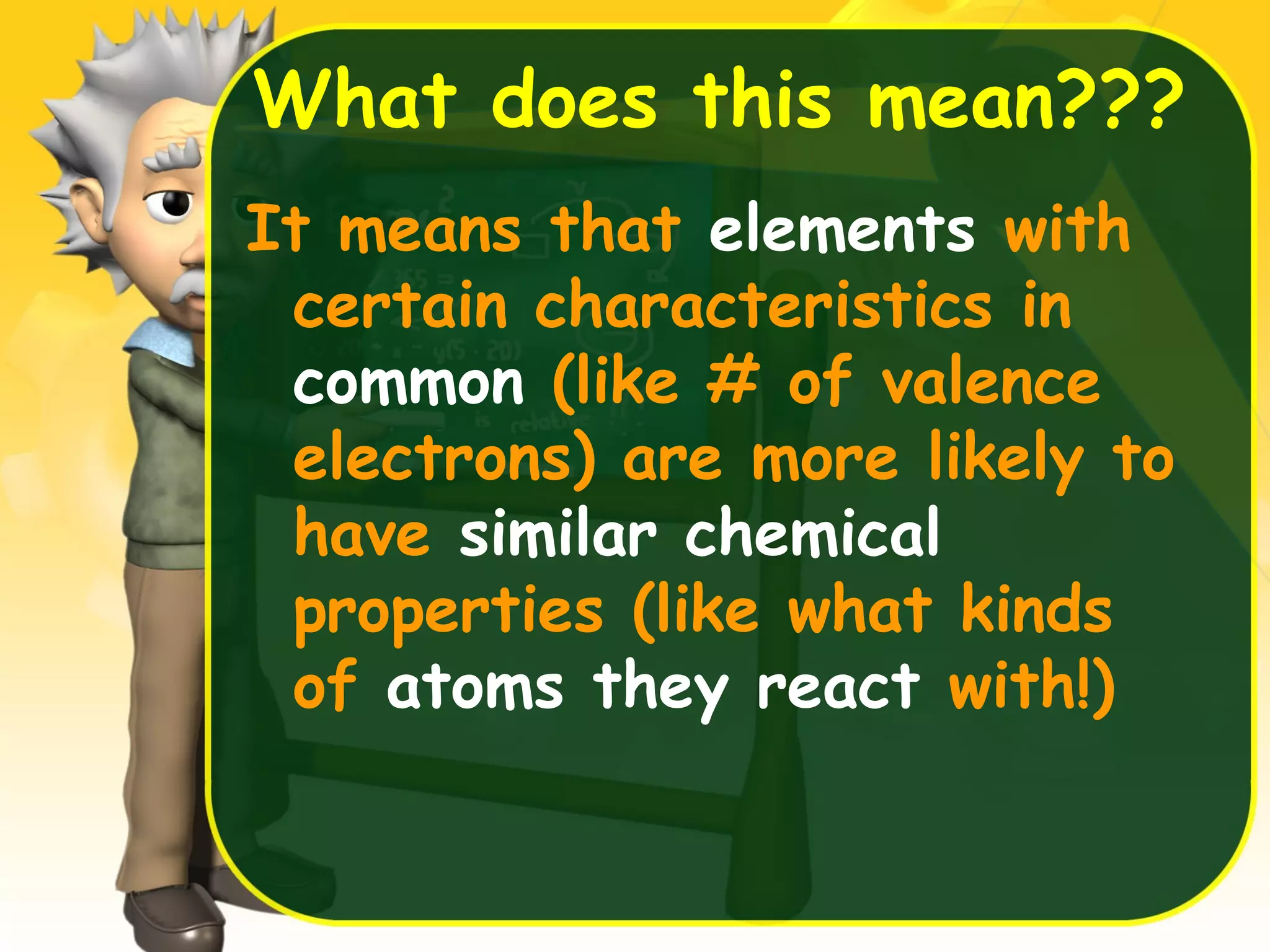
This document provides an overview of atomic structure and the periodic table. It defines protons, neutrons, and electrons, and describes early atomic models including the Bohr model. It introduces electron orbitals and explains how they relate to an element's valence electrons and position on the periodic table. The document then explores periodic trends in atomic properties, including metals, nonmetals, and noble gases. It defines ions, isotopes, and molar mass, and discusses using moles as a unit for counting particles.