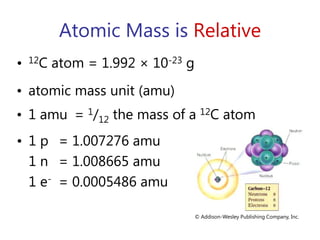
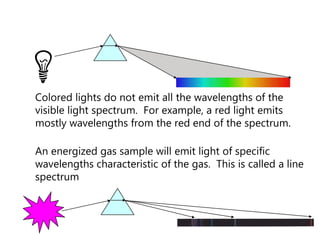
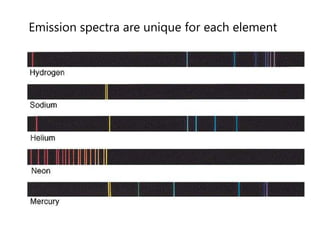
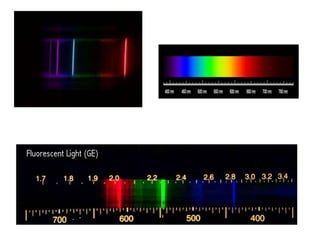
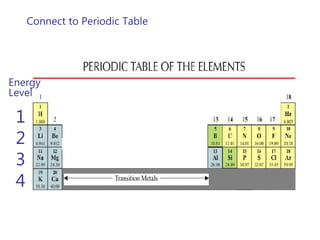
This document discusses atomic structure and provides examples of determining atomic properties. It covers:
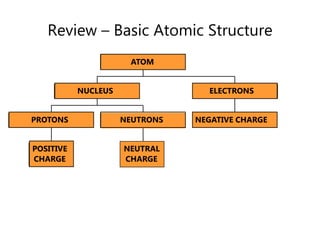
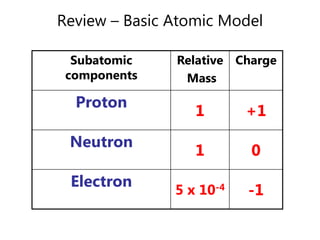
- Basic atomic structure including protons, neutrons, and electrons
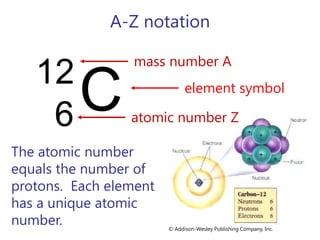
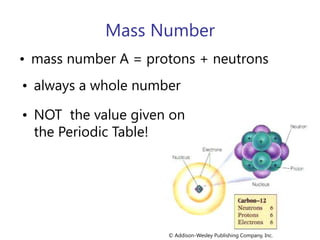
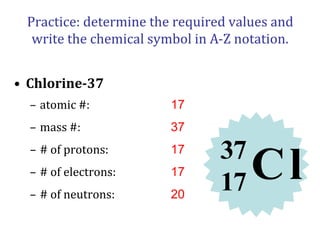
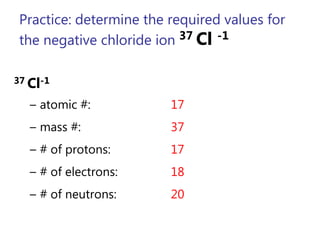
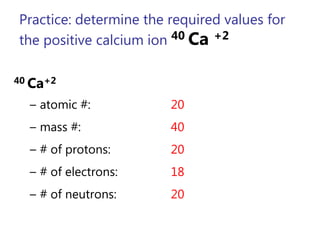
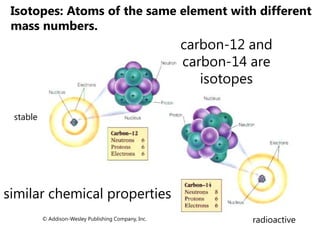
- Atomic number, mass number, and isotopes
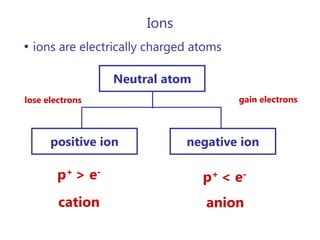
- Ions and their charges
- Uses of radioisotopes
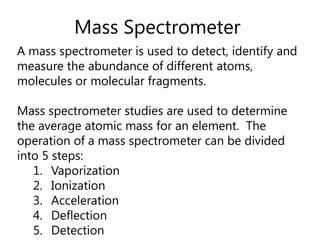
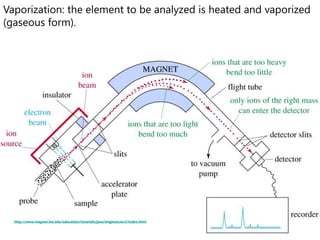
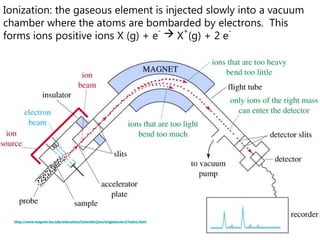
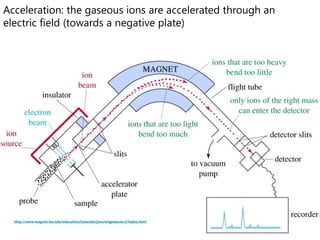
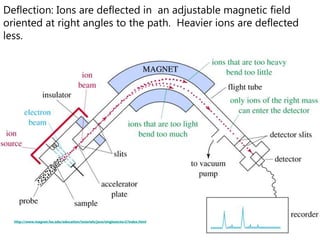
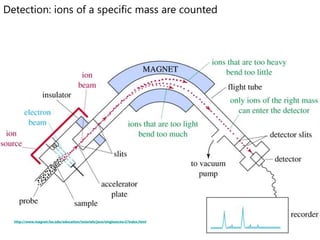
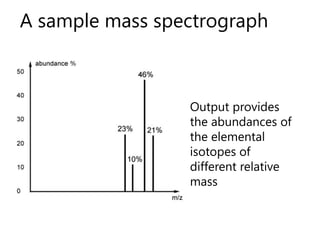
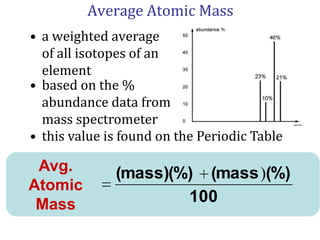
- How a mass spectrometer works to determine atomic masses
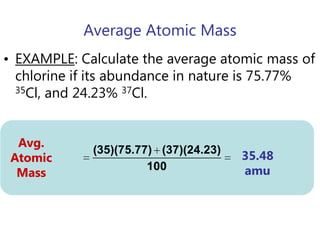
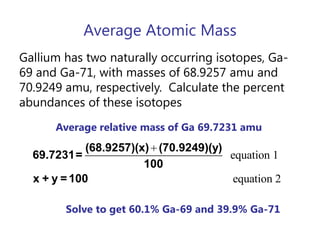
- Average atomic mass calculations
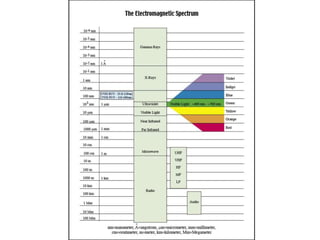
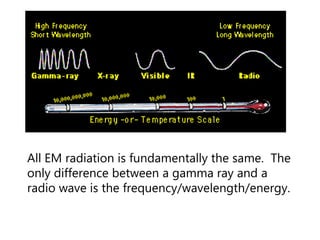
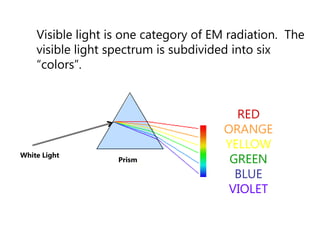
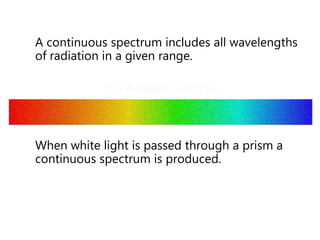
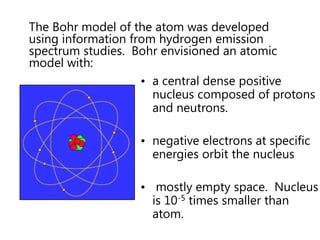
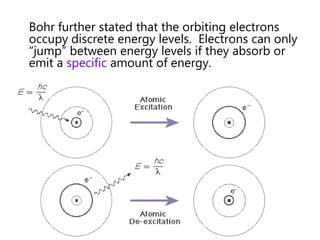
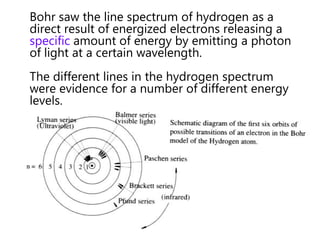
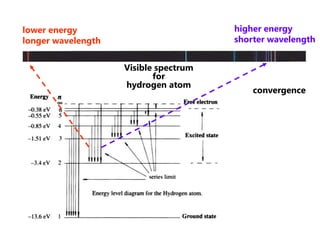
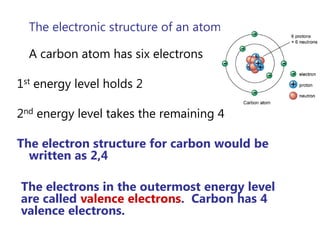
- The Bohr model of the atom and electron configurations