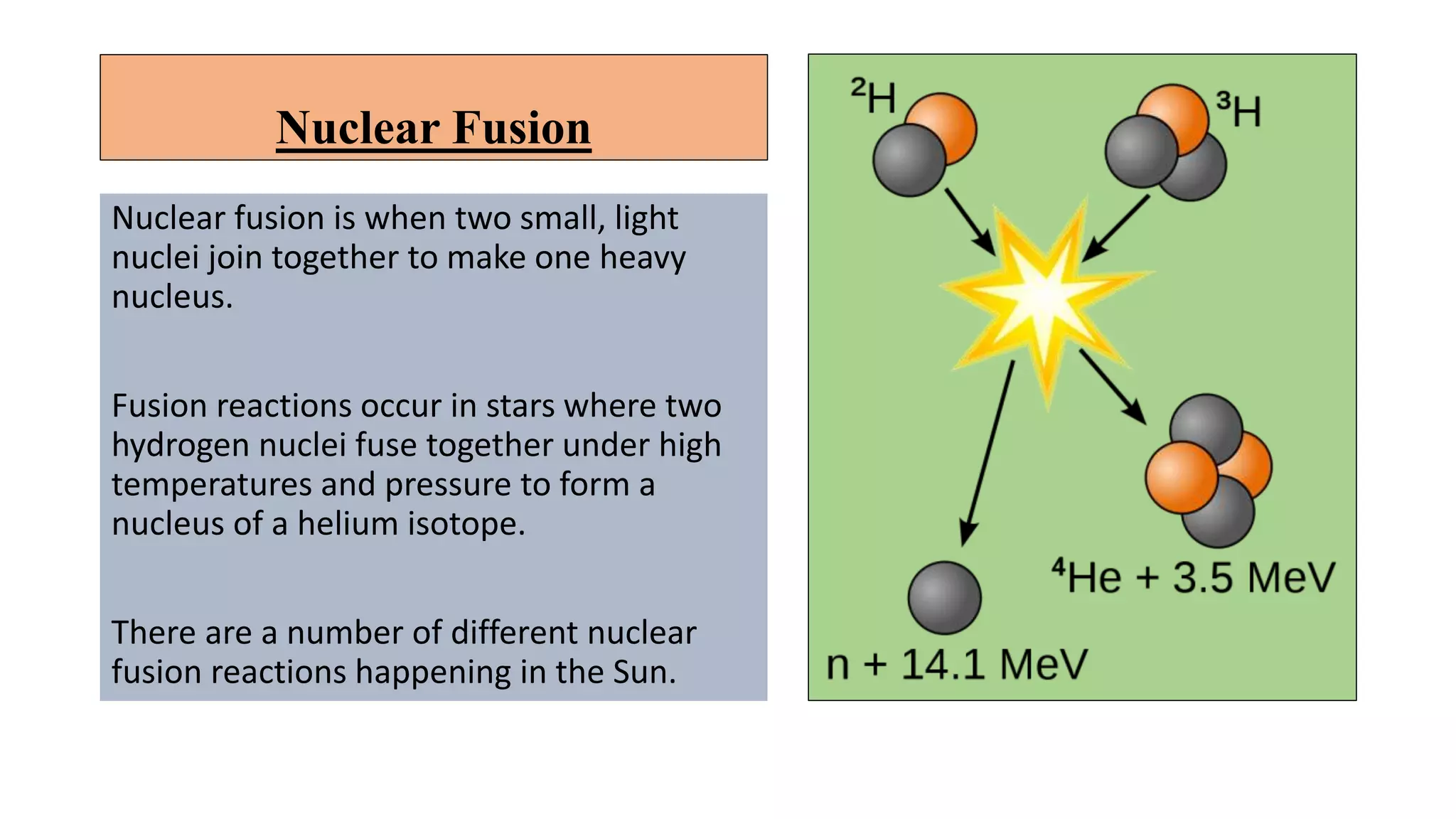
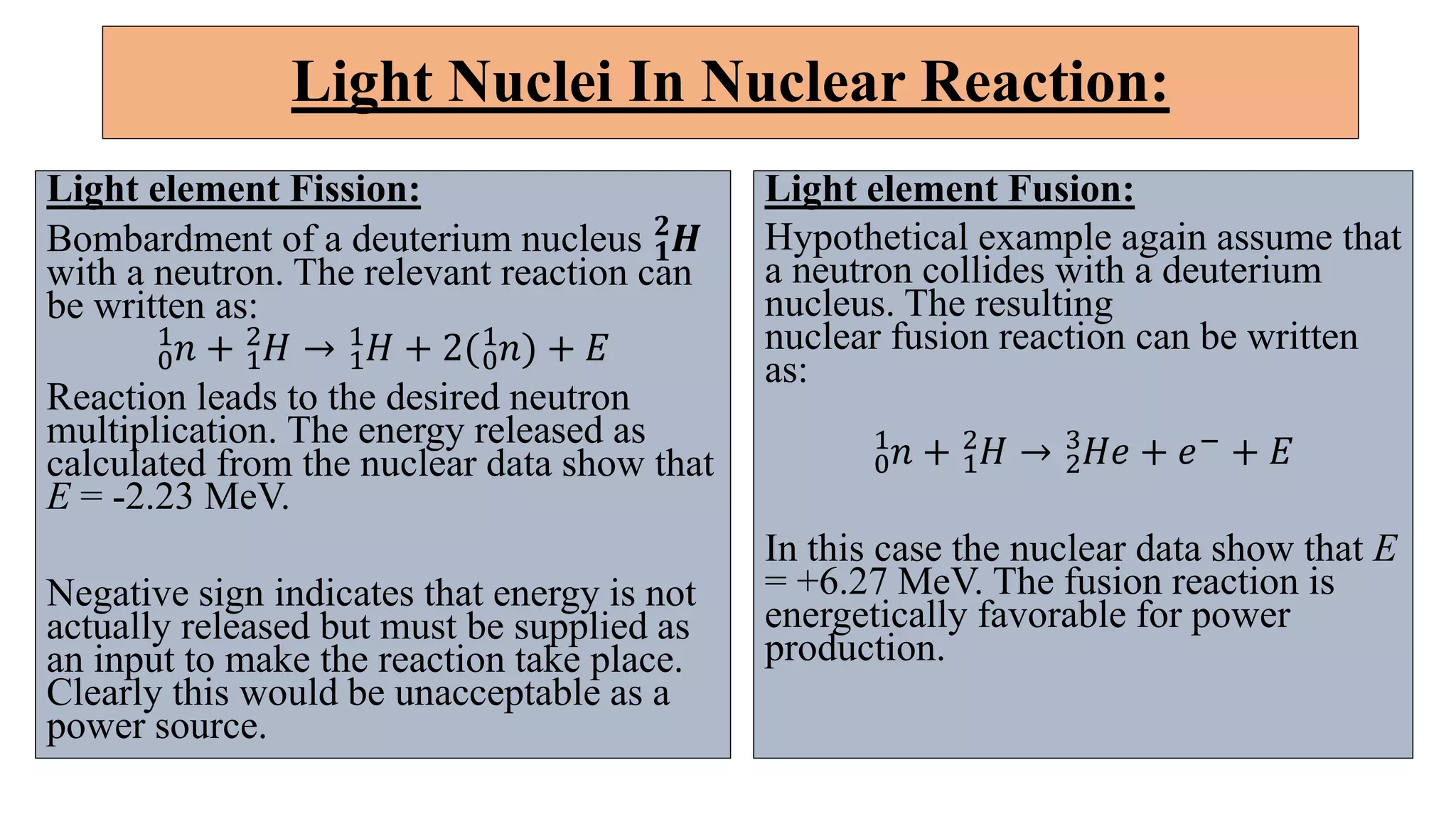
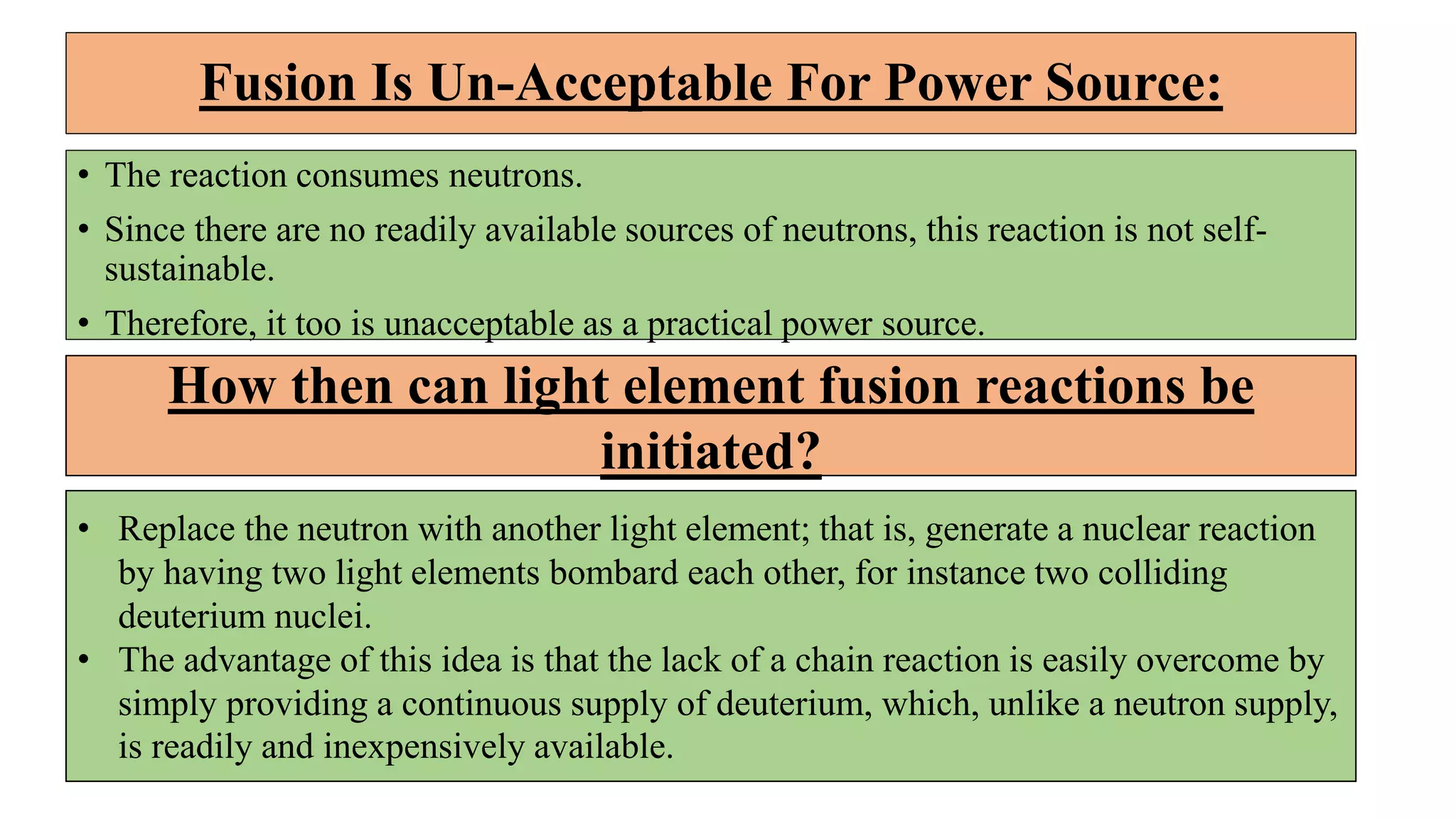
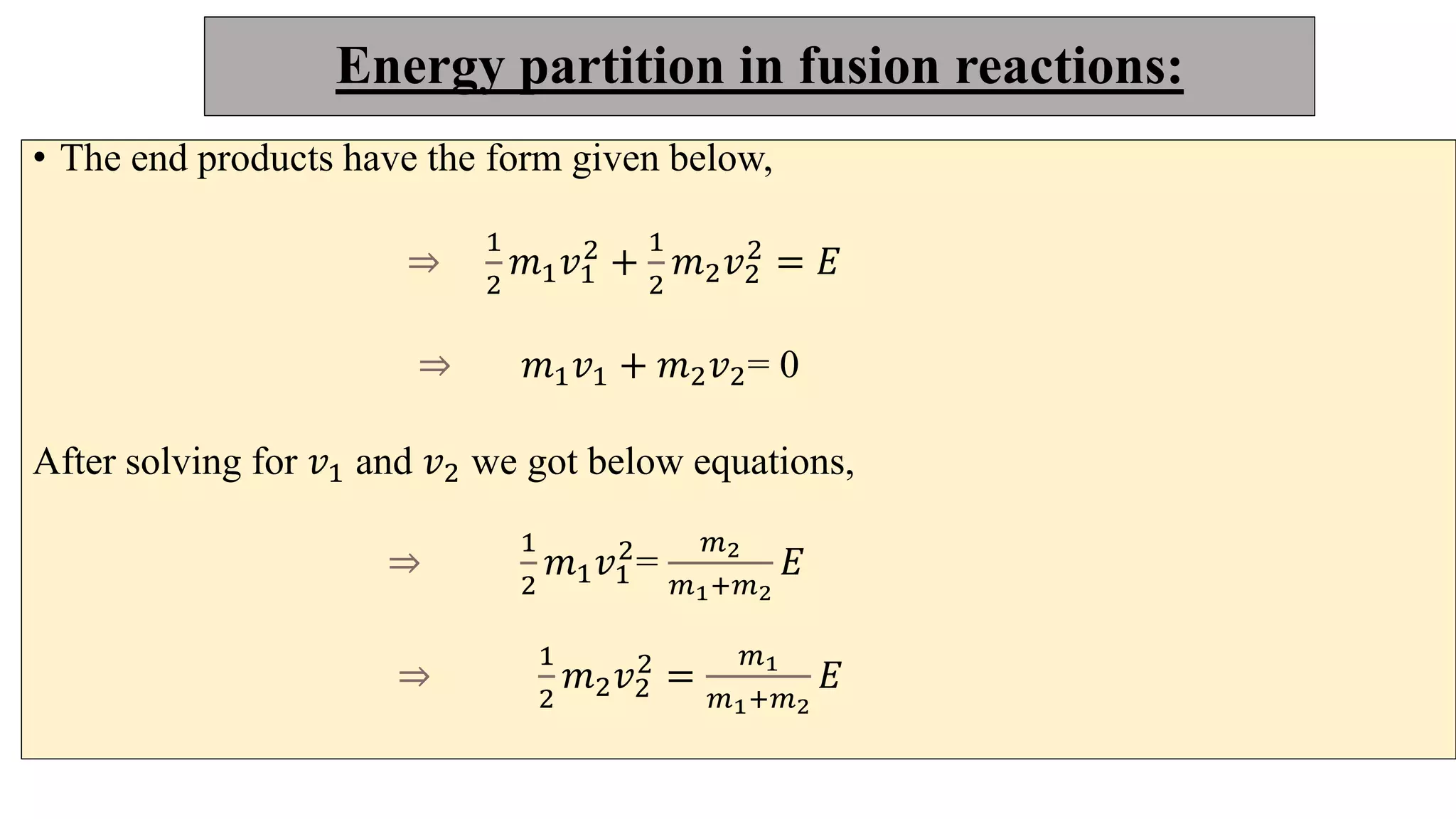
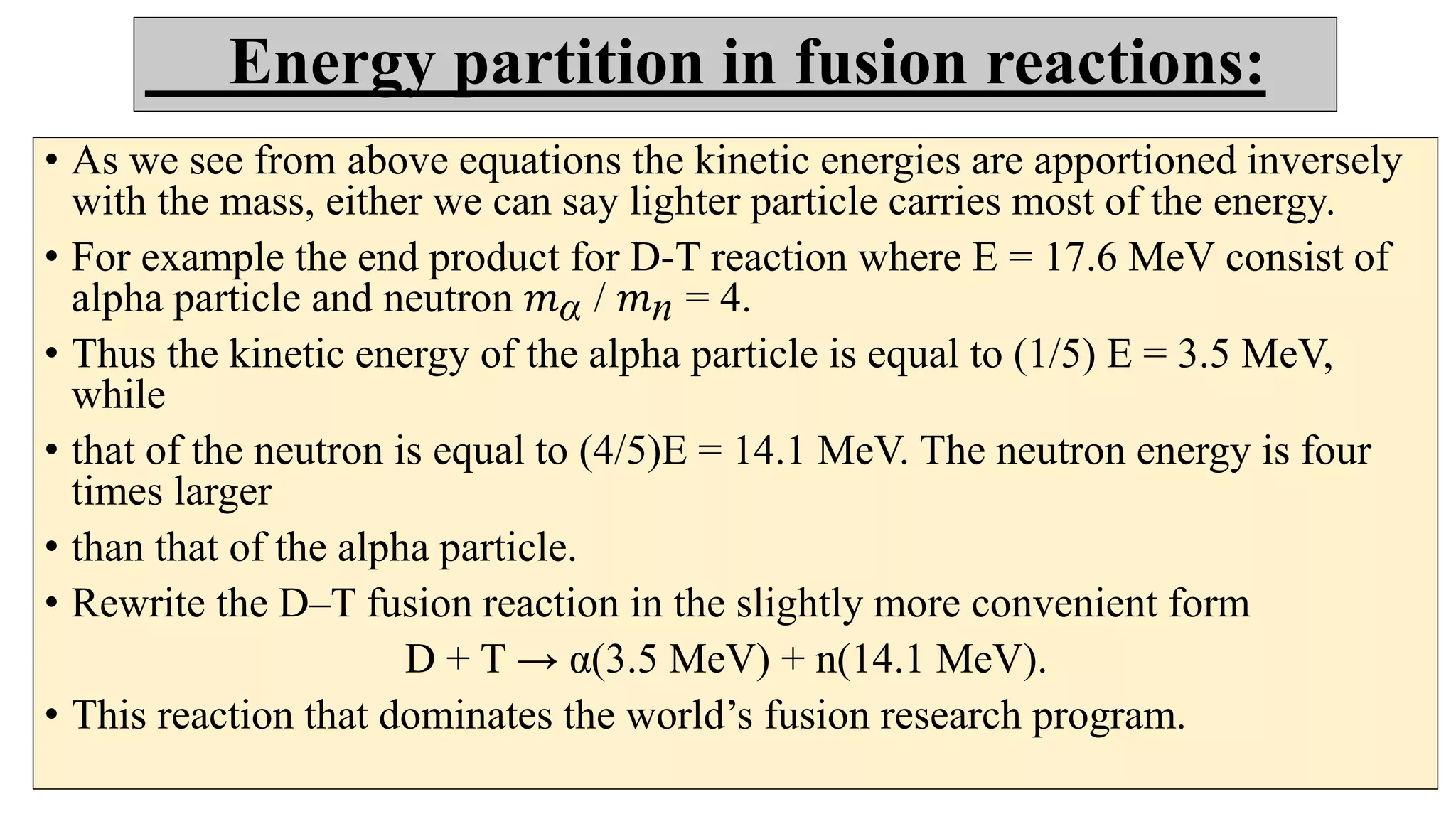
This document discusses nuclear fusion and fission, focusing on their respective reactions and energy outputs. It explains the conditions for nuclear fusion, particularly in stars like the sun, and highlights various reactions involving deuterium and helium isotopes. Additionally, it addresses the challenges and potential of fusion as a power source, including neutron supply issues and energy partitioning in the reaction products.