This document provides an overview of crystal field theory and how it can be used to explain the bonding and spectroscopic properties of transition metal complexes. It discusses how ligands arranged in octahedral, tetrahedral and square planar geometries cause the d-orbitals of the transition metal to split into different energy levels. Factors that influence the size of the crystal field splitting parameter Δo, such as oxidation state, metal identity and ligand type, are also covered.






![Page 10 of 33
For large values of Δo: Δo > P
⇒ complex will be low spin
For small values of Δo: Δo < P
⇒ complex will be high spin
Question 1.1.3
Which of the following compounds has a CFSE of 0.0Δo associated with it?
[PtF6]
[Fe(CN)6]3-
[Mn(OH2)6]2+
Pairing energy - example:
from:
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Crystal_Field_Theory/Virtual%3a_Electronic
_Structure/Ligand_Properties
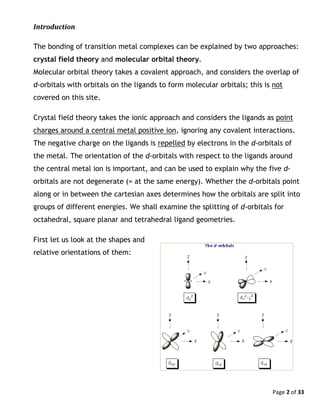
The Fe2+
ion is d6
and has a pairing energy of approximately 17000 cm-1
(this is 2.1
eV or 200 kJ mol-1
). (The pairing energy varies somewhat with the coordination
environment.) For [Fe(H2O)6]2+
, Δo = 10000 cm-1
.
Because P > Δo, [Fe(H2O)6]2+
is a high-spin complex. The electron configuration is
(π*)4
(σ*)2
and the total spin is 2. (Note that water is a weak π-donor ligand.) On
the other hand, for [Fe(CN)6]4-
Δo = 33800 cm-1
, and this complex is low-spin (P <
Δo). All six d electrons reside in the low lying (π) orbitals (cyanide is an excellent
π-acceptor ligand), leading to a total spin of zero.](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-10-320.jpg)
![Page 11 of 33
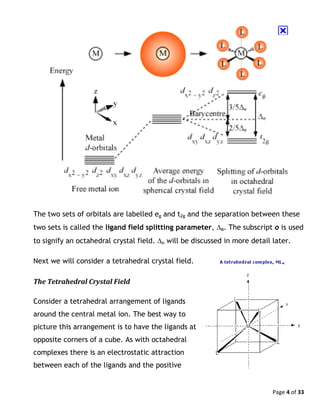
Tetrahedral Complexes
The procedure for working out the CFSEs for tetrahedral complexes is the same
as that for octahedral complexes. However, as the energies of the two set of
orbitals are reversed (the e set is lower in energy than the t2 set) the CFSE for a
t2
x
ey
configuration is now:
CFSE = (-0.6y + 0.4x)Δt
As Δt is less than half the size of Δo, then normally all tetrahedral complexes are
high spin. This is because the pairing energy P is almost always larger than the
splitting between the two energy levels.
Question 1.1.4: Calculate the CFSE of the following molecules - do you expect
high- or low spin configuration ?
[CoCl4]2-
[ReO4]-
[FeCl4]-
1.2 Factors Affecting the Size of Δo
The size of the energy gap between the t2g and eg levels, Δo, depends on 3
factors:
Oxidation state of the transition metal
Row of the transition metal
Ligand](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-11-320.jpg)
![Page 12 of 33
Oxidation State of the Transition Metal
As the oxidation state of the transition metal (effectively the charge on the
metal) is increased, the surrounding ligands are attracted more closely to the
metal centre. The orbitals on the ligands interact more strongly with the d-
orbitals and Δo increases.
Question 1.2.1
Which of the following two complexes would have the smallest value of Δo?
A. [Fe(OH2)6]3+
B. [Fe(OH2)6]2+
Row of the Transition Metal
On going from the first to the second row of a transition metal triad, there is
approximately a 50% increase in the size of Δo, and another 50% increase on going
from the second row to the third row. This is due to the fact that as you descend
a transition metal triad the size of the d-orbitals increases (3d < 4d < 5d). The
larger d-orbitals interact more with the orbitals on the ligands, hence Δo is
larger. It should also be noted that the pairing energies for the larger d-orbitals
are smaller, which means that low spin configurations are favoured.
Question 1.2.2
Which of the following would have the smallest value of Δo?
A. [Os(OH2)6]3+
B. [Fe(OH2)6]3+
C. [Ru(OH2)6]3+](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-12-320.jpg)
![Page 13 of 33
Ligand
The size of Δo depends on the ligand(s) present according to the spectrochemical
series, which is a list of ligands in order of decreasing crystal field strength.
CN-
= CO = C2H4 > PR3 > NO2
-
= phen > bipy > SO3
2-
> en = py = NH3 > edta4-
> NCS-
> H2O > C2O4
2-
> ONO2
-
> OSO3
2-
> OH-
= ONO-
> F-
> Cl-
= SCN-
> Br-
> I-
Weak field ligands, such as Cl-
and SCN-
, give rise to small values for Δo, while
strong field ligands, such as CN-
and CO, give rise to large values for Δo.
It is also possible to arrange the metals according to a spectrochemical series as
well. The approximate order is:
Mn2+
< Ni2+
< Fe2+
< V2+
< Fe3+
< Co3+
< Mn3+
< Mo3+
< Rh3+
< Ru3+
< Pd4+
< Ir3+
< Pt4+
Question 1.2.3
Which of the following would have the largest value of Δo?
A. [Cr(OH2)6]3+
B. [CrF6]3-
C. [Cr(NH3)6]3+
D. [Cr(NH3)6]2+
That concludes the section on factors affecting the size of Δo.](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-13-320.jpg)
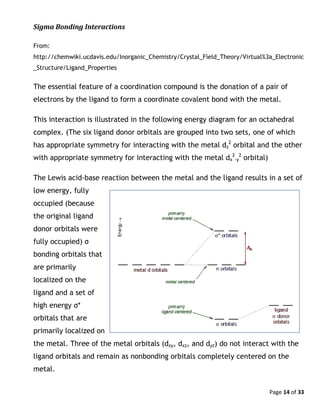
![Page 15 of 33
The d-orbital splitting, Δo, arises from the increase in energy of the σ* orbitals
relative to the nonbonding orbitals. The stronger the bonding interaction, the
higher the σ* energy and the greater Δo. A strong σ bonding interaction requires a
good energy match between the metal and the ligand. Empirically, there is a
correlation between Δo and the basicity of the ligand. A ligand that is a good
Brønsted (or Lewis) base tends to form strong σ bonds with metals and thus
produces a relatively large Δo.
Pi Bonding Interactions
Some ligands are capable of π bonding interactions, either by acting as a π
acceptor ([Cr(CO)], for example) or a π donor ([Cr(F)]2+
, for example). The energy
diagram shown below illustrates the behavior of a π donor ligand on Δo.](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-15-320.jpg)
![Page 16 of 33
1.3 The Jahn-Teller Effect
This section will include:
Jahn-Teller theorem
Example of complexes
Nature of the distortion
Explanation of why it occurs
Jahn-Teller Theorem
In 1937 H.A. Jahn and E. Teller put forward a theorem which explained some of
the distortions observed in transition metal complexes. This became known as
the Jahn-Teller theorem. It states:
"For any non-linear molecule in an electronically degenerate state, distortion
must occur to lower the symmetry, remove the degeneracy and lower the
energy."
These distortions are called Jahn-Teller distortions. But how are the molecules
distorted, and how does the distortion occur? This section aims to describe what
is meant by an electronically degenerate state and explain how Jahn-Teller
distortions can lower the energy of molecules in such states.
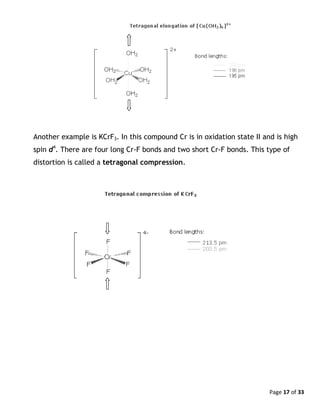
Examples of distorted complexes
There are some complexes which might at first be expected to be octahedral, but
which are in fact distorted from the ideal octahedron. These distortions are
called Jahn-Teller distortions. One notable example is the complex ion
[Cu(OH2)6]2+
, shown below. Two of the metal-ligand bonds are longer than the
other four. This type of distortion is called a tetragonal elongation.](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-16-320.jpg)
![Page 18 of 33
For comparison, below is the [Ni(OH2)6]2+
complex ion, which is symmetrical and
undistorted:
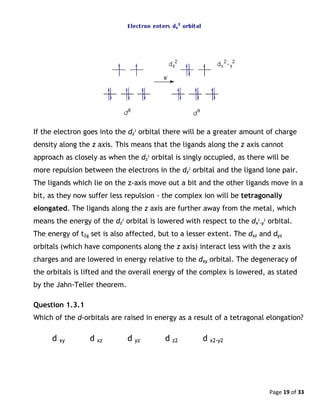
Explanation
The existence of distorted complexes, such as [Cu(OH2)6]2+
and KCrF3 (shown on
the previous page), can be explained by considering their electronic structure.
Consider the complex ion [Cu(OH2)6]2+
, which is a tetragonally elongated
octahedron. Cu(II) is d9
and the population of the d orbitals is t2g
6
eg
3
. The third
electron in the eg set may occupy either the dz
2
orbital or the dx
2
-y
2
orbital, as
they are equal in energy. This is what is meant by an electronically degenerate
state.
Which orbital will the ninth electron go into?](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-18-320.jpg)

![Page 21 of 33
Elongation results in a greater stabilization than compression; this explains why
the [Cu(OH2)6]2+
ion is tetragonally elongated.
Jahn-Teller distortions will occur for any electronically degenerate configuration,
not just for d9
. If there is a choice of where to put the electrons in a complex,
then there will be a Jahn-Teller distortion to remove this choice.
Example: [Ti(OH2)6]3+
: Ti is in oxidation state III. Ti is d1
, and the electron can
occupy the dxz, dyz or the dxy orbital.
The complex will undergo a Jahn-Teller distortion to remove this choice.
Tetragonal compression is likely because only one d-orbital has lowest energy !](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-21-320.jpg)


![Page 25 of 33
2. UV-visible absorption spectroscopy
This section will include:
Example spectra
Explanation of key features of the
spectra
Using spectra to calculate values of Δo
Selection rules
How we see colours
White light (simplified by showing only green, red, and blue) is shown through a
solution. The solution absorbs the red and green wavelengths, however the blue
light is reflected and passes through, so we see the solution as being blue:
Absorption Spectra of Transition Metal Complexes
Solutions of transition metal complexes come in a variety of colours. For
example, a solution of [Cu(OH2)6]2+
is blue, a solution of [MnO4]-
is an intense
purple, and a solution of [Co(OH2)6]2+
in water is pink. In contrast, [Zn(OH2)6]2+
is
colourless. What is responsible for the difference in colour?
Complexes are coloured because they absorb light in the visible region. The
colour observed (or transmitted) is complementary to the colour of the light
absorbed. If light from the yellow-green part of the spectrum is absorbed, the
colour observed will be white light with yellow-green subtracted out, that is,
violet.
Wavelengths of colours
and complementary colours](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-25-320.jpg)
![Page 26 of 33
Colour of light
absorbed
Wavelength
ranges / nm
Colour of light
transmitted
Red 700-620 Green
Orange 620-580 Blue
Yellow 580-560 Violet
Green 560-490 Red
Blue 490-430 Orange
Violet 430-380 Yellow
Absorption spectra of transition metal complexes
The absorption bands in spectra are a result of HOMO-LUMO transitions. The
HOMO and LUMO of an octahedral complex are mostly d-orbital in character (the
separation between the two is characterised by the size of Δo), so these
transitions are called d-d transitions. It is these electronic transitions that are
responsible for the colour of complexes.
The pages that follow use the complex [Ti(OH2)6]3+
to demonstrate how the value
of Δo for a complex can be calculated from its absorption spectrum. The selection
rules for electronic d-d transitions are also considered.
UV-Visible Spectrum of
[Ti(OH2)6]3+
Ti is in oxidation state III and
is d1
. The metal has the
electronic configuration
t2g
1
eg
0
.
A d-d transition takes place
(t2g
0
eg
1
← t2g
1
eg
0
) which is](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-26-320.jpg)

![Page 28 of 33
Another common way to express the CFSE is in cm-1
(1 kJ/mol = 84 cm-1
),
in this example about 20.400 cm-1
which corresponds to a wavelength of 490 nm.
You should be familiar with energy conversions (convert between kJ and kJ/mol
and cm-1 and wavelength in nm) !
Additional features of the UV-visible spectrum of [Ti(OH2)6]3+
The absorption band for the d-d transition has a shoulder
This is due to Jahn-Teller distortion, a tetragonal compression. The splitting
diagram for a tetragonal compression below, shows that there are two possible d-
d transitions from the t2g to the eg. This means that light of two different
wavelengths is absorbed and there are two different absorption peaks. The two
peaks are close together and one appears as a shoulder on the other.](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-28-320.jpg)
![Page 29 of 33
The absorption band for the d-d transition is broad
This is due to the fact that molecules are not static. The [Ti(OH2)6]3+
molecule is
continually vibrating and the Ti-O bond lengths are continually changing. The size
of Δo depends on the Ti-O bond length. If the Ti-O bonds are longer there is less
repulsion between the ligand lone pairs and the d-electrons, so Δo will be
smaller. At any one instant, there will be a range of Ti-O bond lengths in solution
and a range of Δo values. Hence the absorption band is broad.
There is a strong absorption in the UV region (to the left of the graph)
This is due to charge transfer transitions. This type of transition involves
electrons moving from orbitals which are essentially ligand in character to
orbitals which are essentially metal in character. This means charge transfer
from one part of the molecule to another takes place. This type of absorption
tends to be intense as there are no selection rules governing the transition.
(Explanation about metal- and ligand-character of orbitals will follow in another
part about Ligand-Field Theory or MO-Theory !)
Charge transfer transitions can also take place in the visible region of the
electromagnetic spectrum. When this happens, the complex is usually intensely
coloured. For example, the complex KMnO4 is a deep purple colour because
charge transfer transitions take place between O2-
ligands and the Mn metal
centre (ligand → metal charge transfer). Metal → ligand charge transfer is also
possible.](https://image.slidesharecdn.com/crystalfieldtheoryv2-140626101519-phpapp01/85/Crystal-field-theory-29-320.jpg)