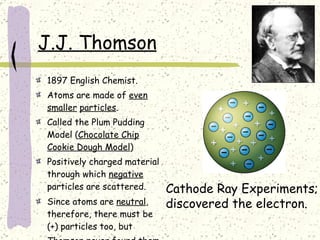
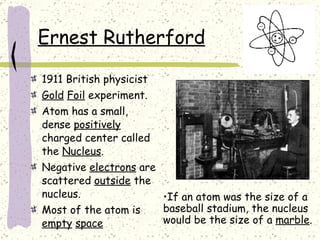
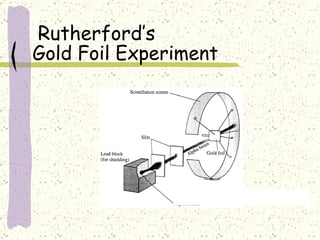
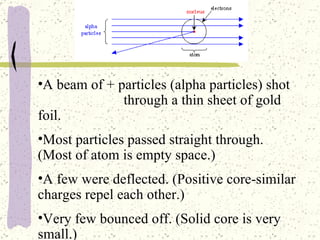
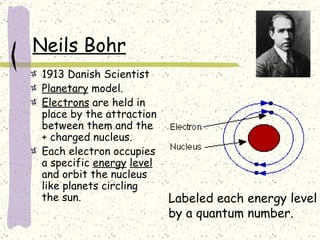
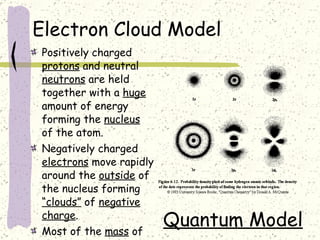
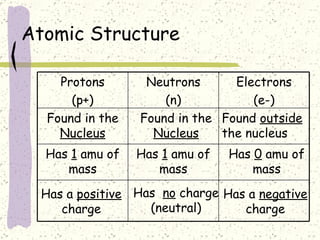
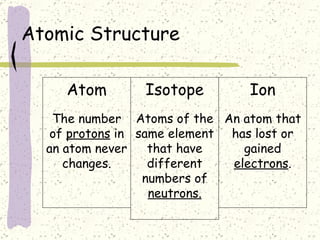
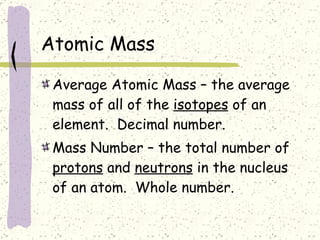
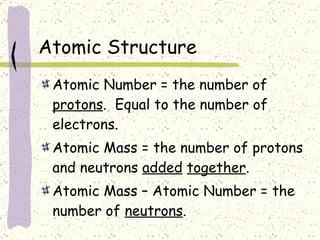
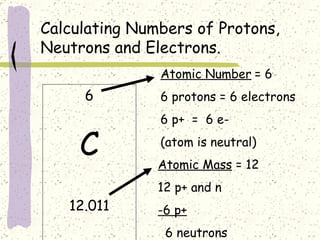
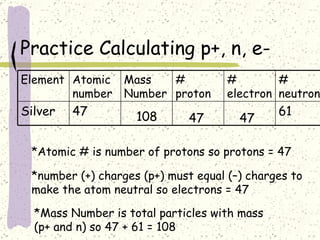
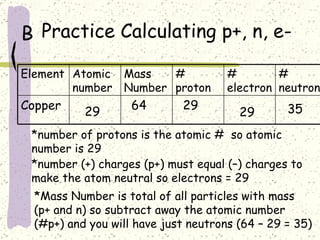
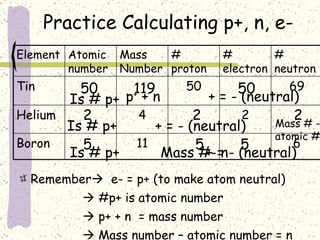
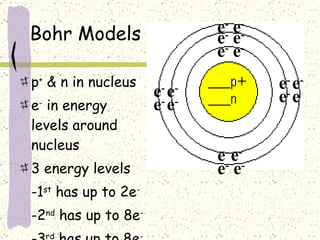
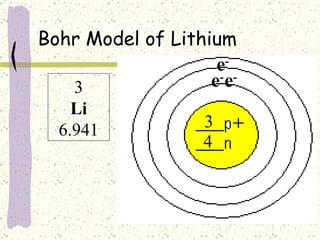
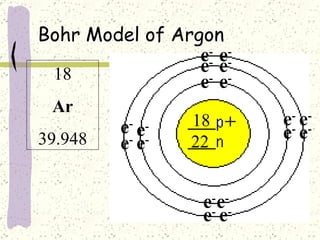
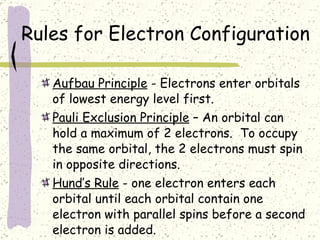
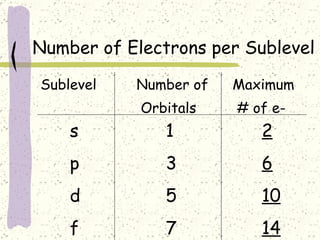
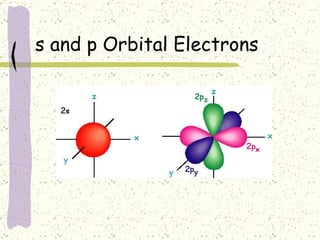
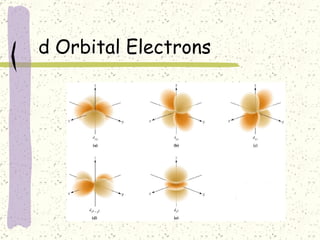
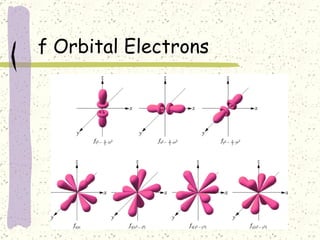
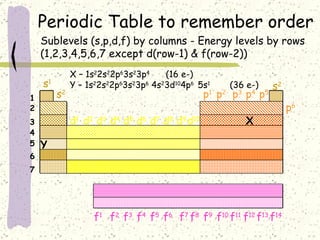
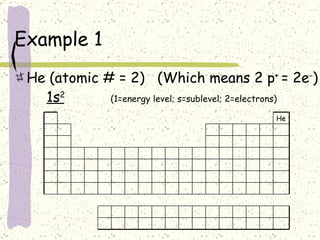
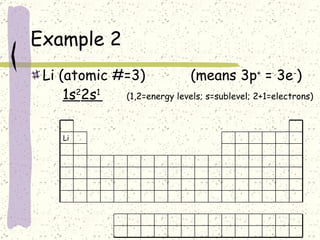
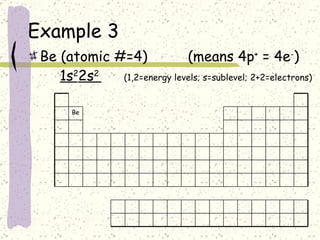
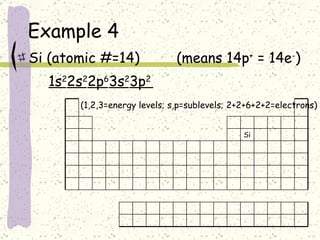
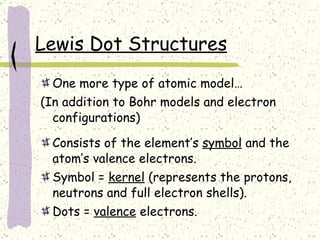
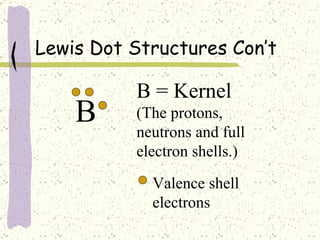
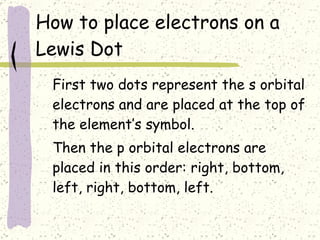
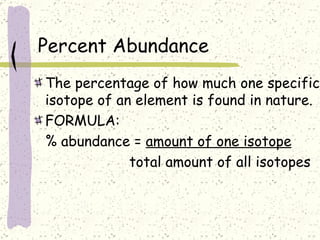
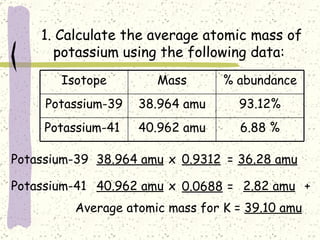
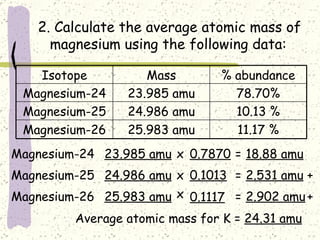
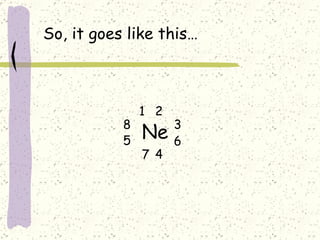This document provides a brief history of the atomic theory, from early philosophers' ideas to the modern quantum model. It describes Democritus' idea of indivisible atoms, Dalton's model of atoms as solid spheres, Thomson's discovery of electrons, Rutherford's gold foil experiment showing the nucleus, Bohr's planetary model, and the modern electron cloud/quantum model with protons and neutrons in the nucleus and electrons in clouds outside. It also discusses atomic structure, isotopes, ions, atomic mass, and calculating atomic properties using atomic number and mass number.