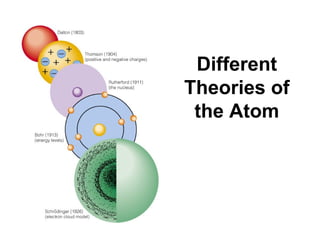
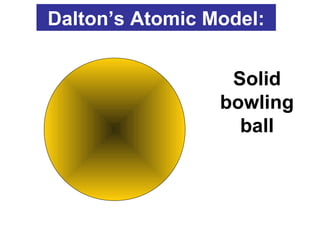
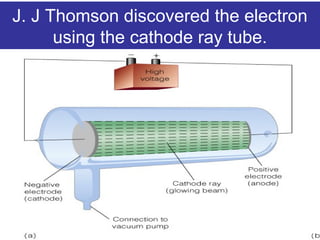
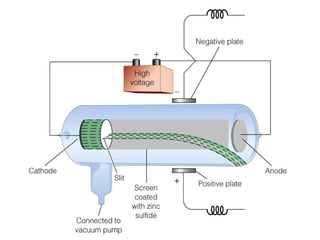
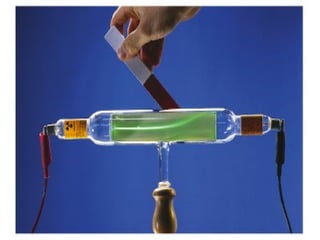
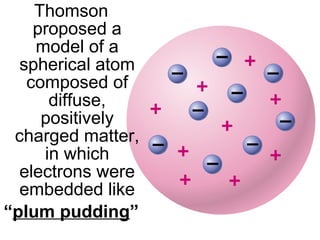
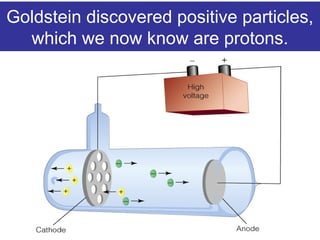
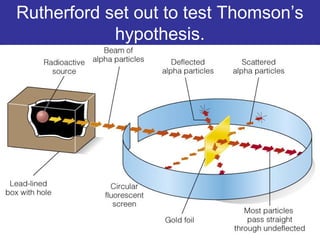
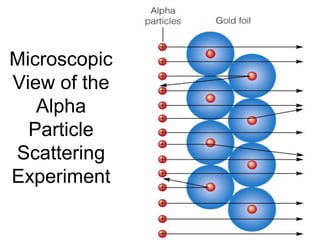
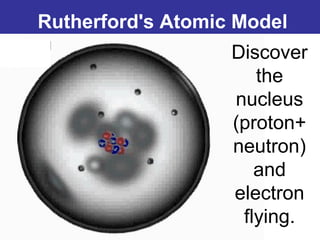
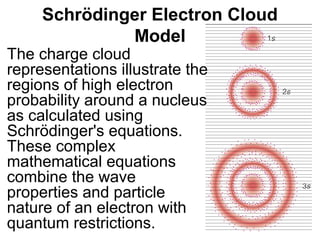
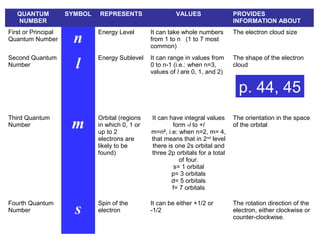
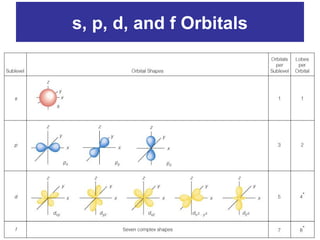
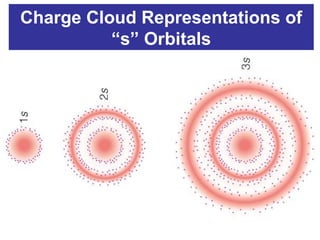
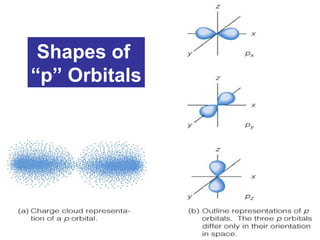
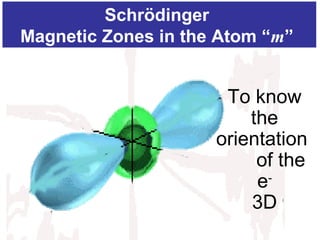
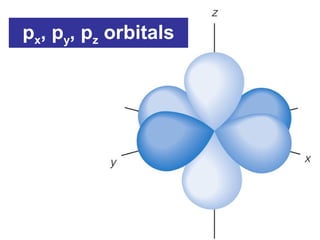
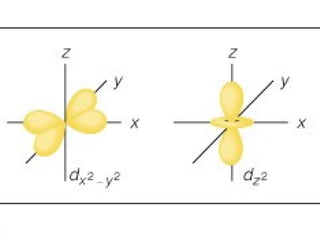
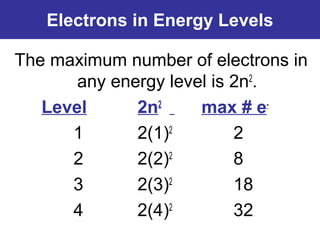
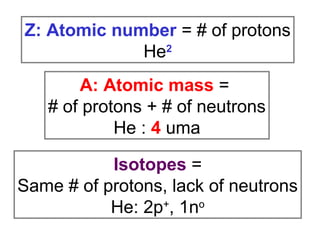
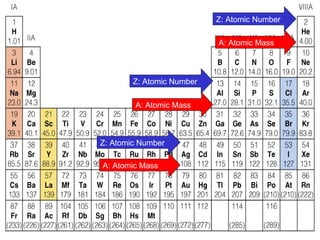
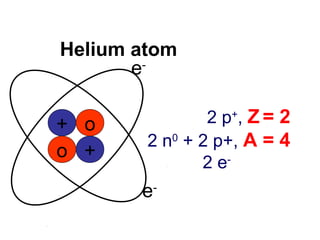
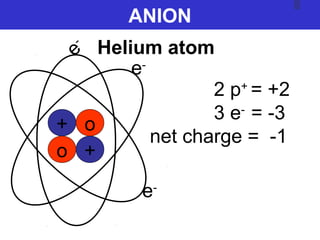
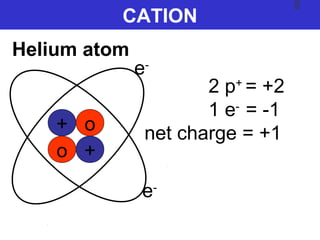
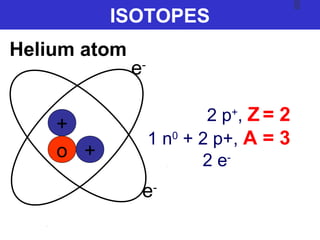
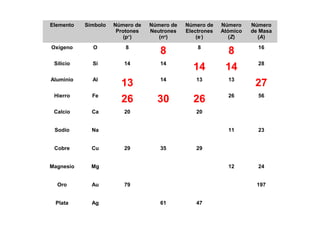
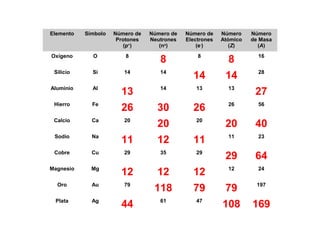
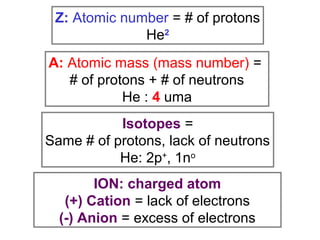
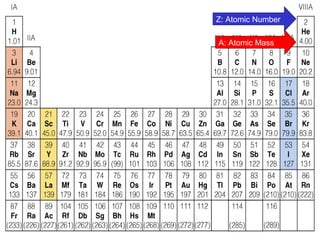
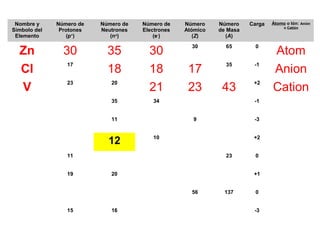
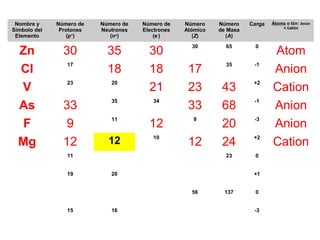
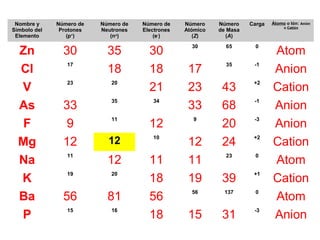
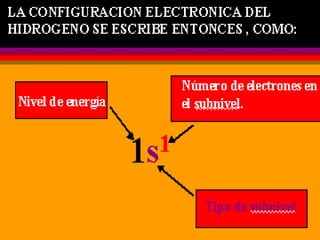
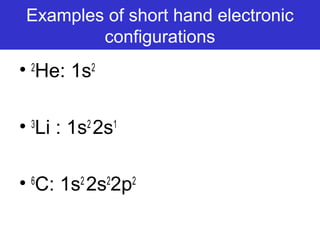
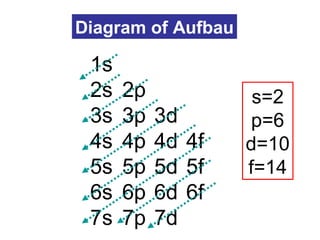
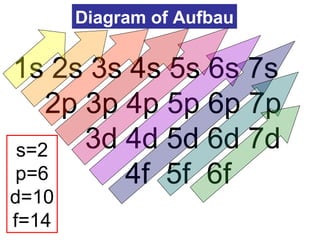
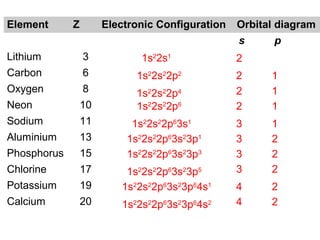
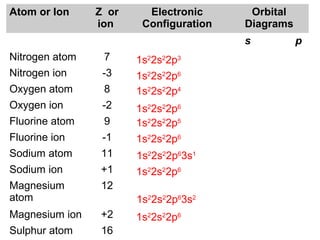
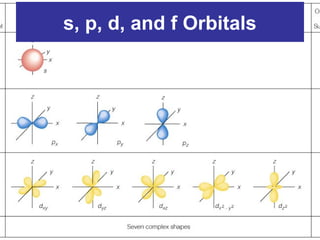
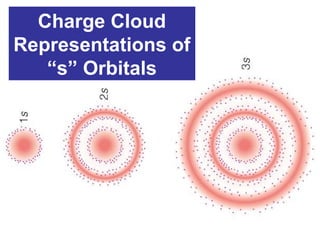
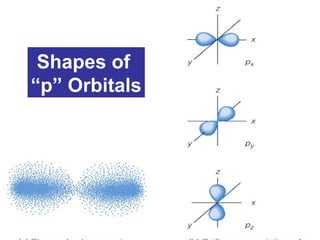
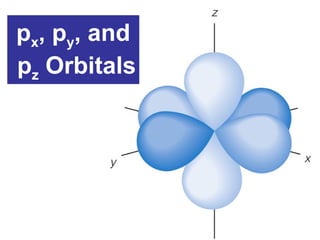
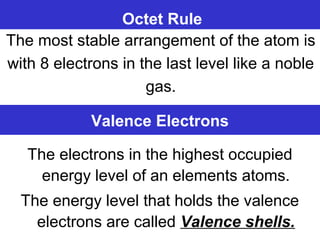
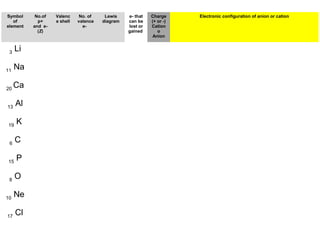
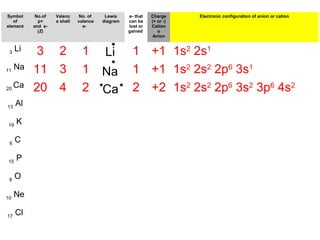
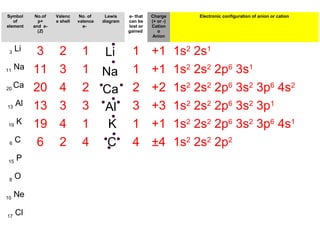
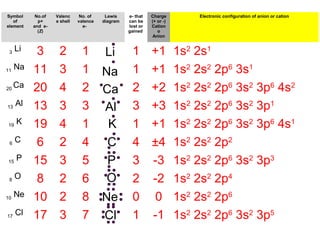
The document discusses the history and development of atomic theory from ancient Greek philosophers to modern quantum mechanics. It summarizes key contributors and their models including Dalton's atomic theory, Thomson's plum pudding model, Rutherford's nuclear model, Bohr's planetary model, Schrödinger's wave mechanical model, and the modern quantum mechanical model incorporating quantum numbers. Diagrams illustrate many of the historic atomic models.