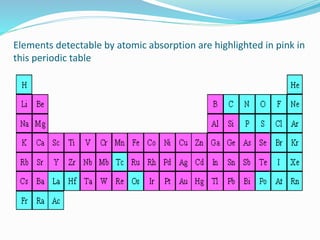
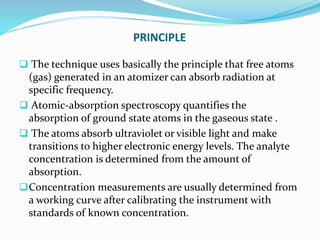
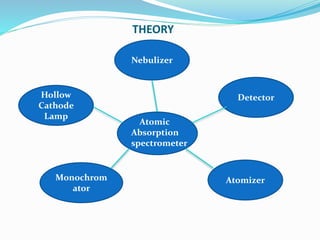
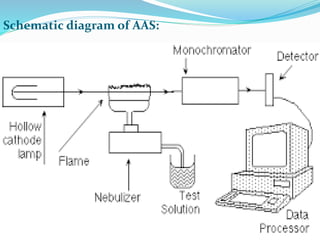
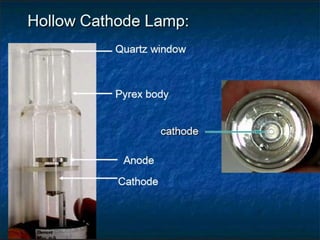
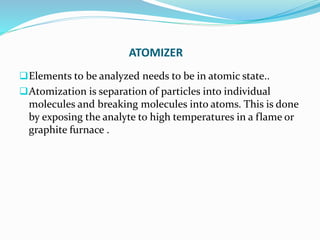
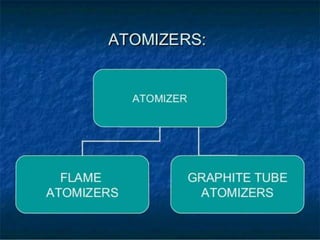
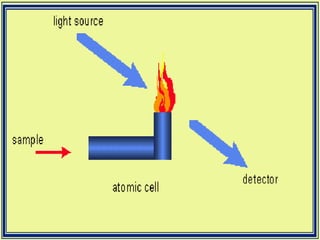
Atomic absorption spectroscopy is a technique used to detect metals and metalloids in samples. It works by atomizing the sample using a flame or graphite furnace and measuring the absorption of light from a hollow cathode lamp at specific wavelengths. Key components of the instrumentation include the lamp, atomizer, monochromator and detector. It can be used to analyze over 62 elements and is applied in areas such as environmental analysis, the food industry and pharmaceutical analysis.