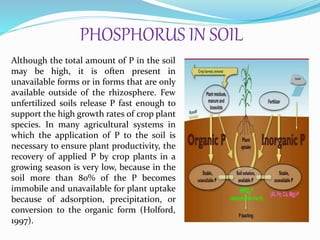
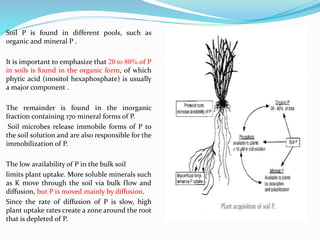
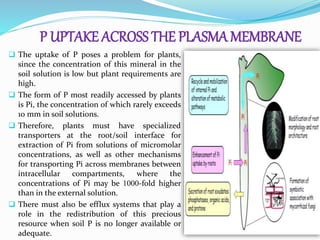
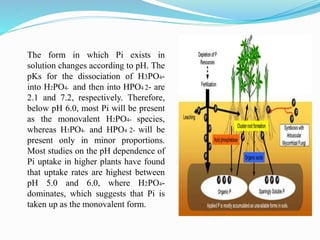
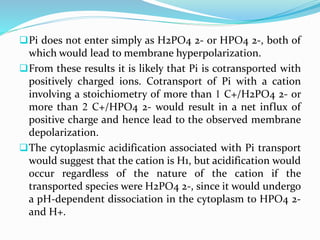
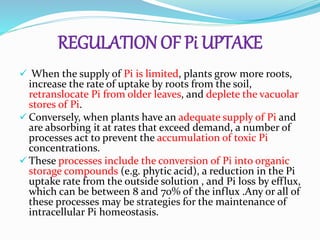
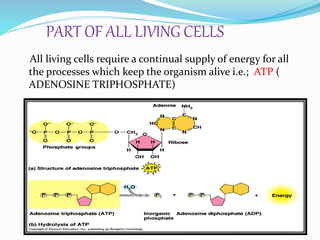
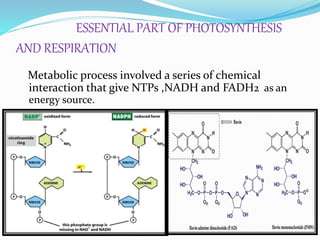
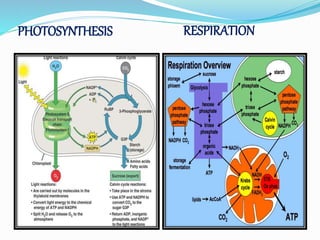
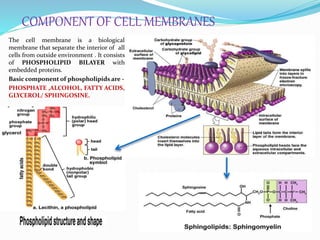
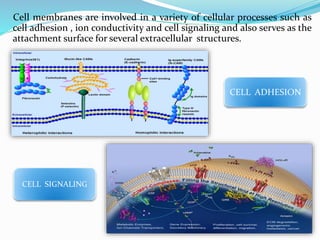
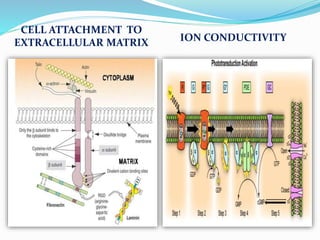
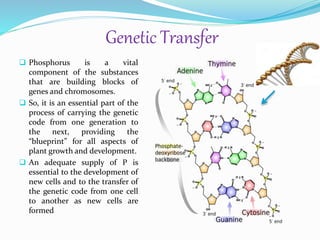
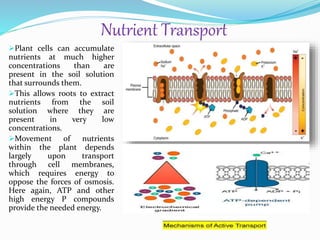
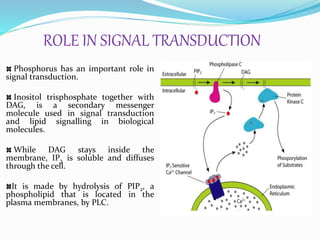
Phosphorus is an essential plant macronutrient that is required for many critical cellular functions and processes. It is a component of key molecules like nucleic acids, phospholipids, and ATP. Phosphorus exists in both organic and inorganic forms in soil, but most soil phosphorus is unavailable to plants. Plants have developed strategies to acquire phosphorus from soil like forming specialized root structures and exuding organic acids. Phosphate is transported across plant membranes through cotransporters and is compartmentalized within cells. Plants tightly regulate phosphorus uptake, transport, and recycling in response to phosphorus availability through physiological and morphological adaptations.