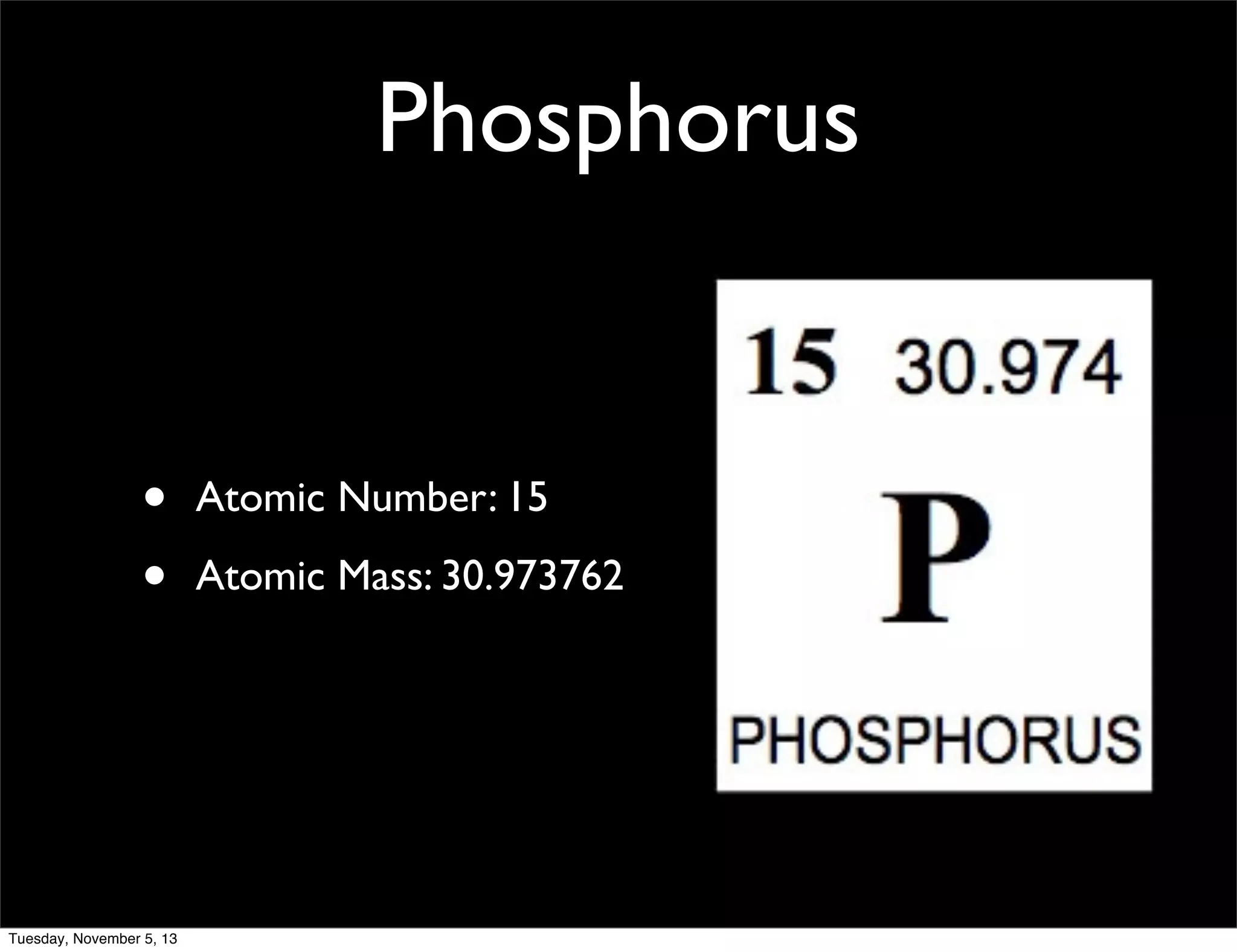
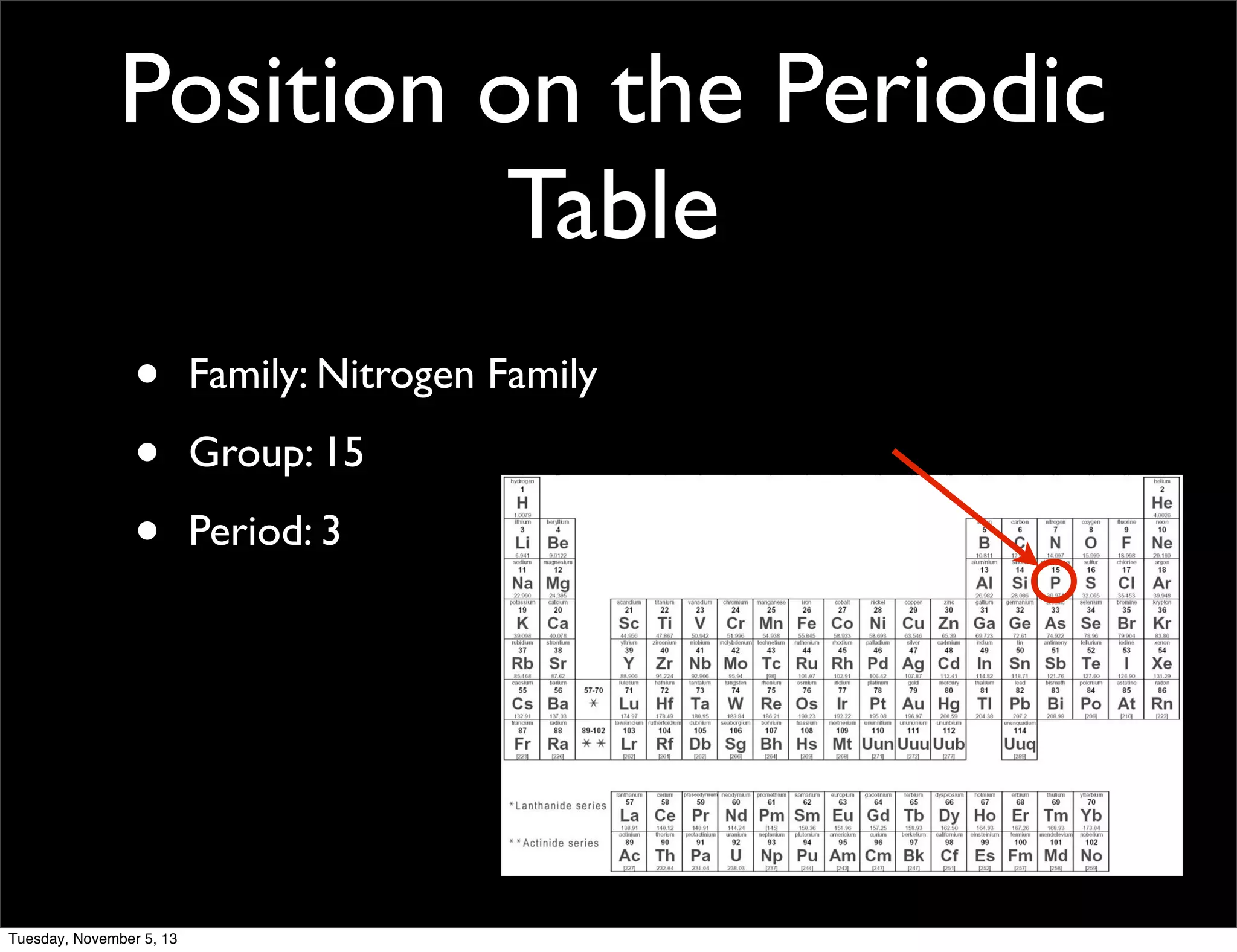
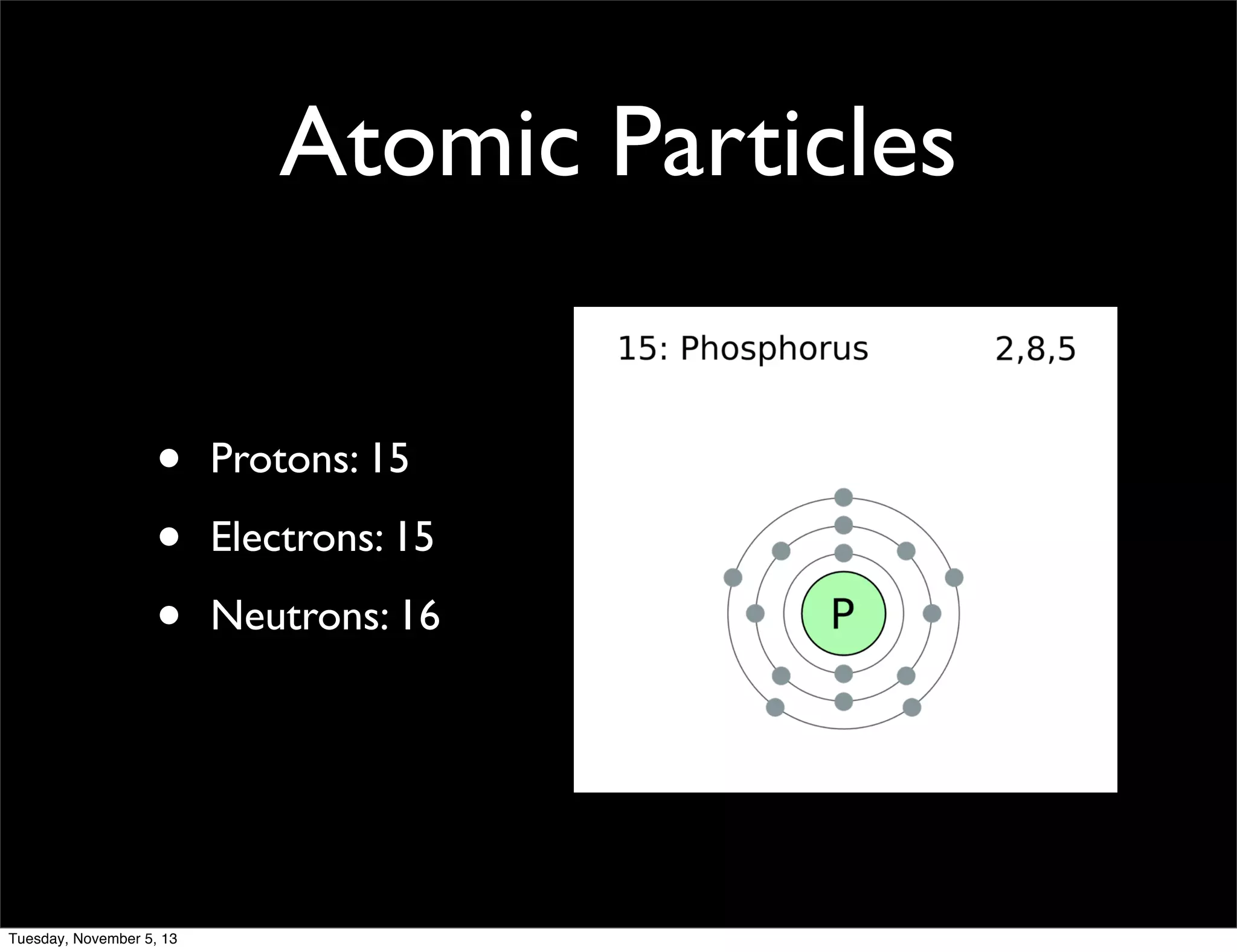
Phosphorus is a non-metal that exists in two forms, white and red. It has an atomic number of 15 and atomic mass of 30.97. Phosphorus was discovered in 1669 by Hennig Brand through the distillation of urine. Phosphorus combines with calcium to form calcium phosphate, which gives strength to bones and teeth. It is widely used in matches and water softening due to its flammability.