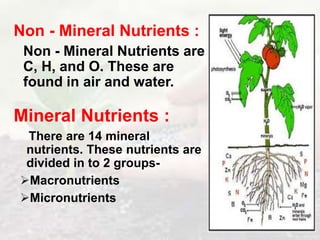
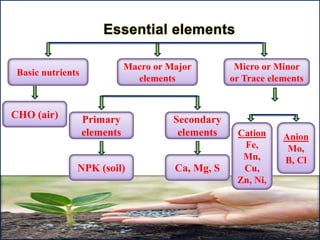
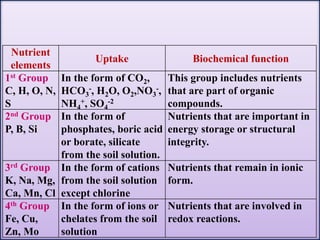
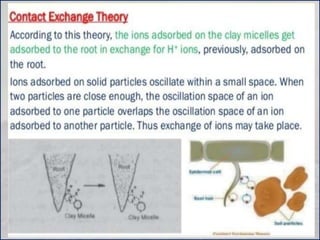
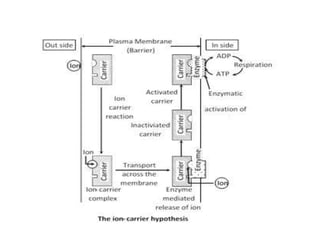
The document discusses plant nutrition and the classification of nutrient elements. It states that 17 chemical elements are important for plant growth and survival. These elements can be divided into non-mineral nutrients (C, H, O) and mineral nutrients, with the latter further divided into macronutrients and micronutrients based on the quantity needed. Macronutrients include nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur. Micronutrients are needed in very small quantities and include manganese, chlorine, copper, iron, molybdenum, and zinc. The document also discusses the criteria for essentiality of elements, classification based on mobility, and the mechanisms of nutrient uptake in plants.