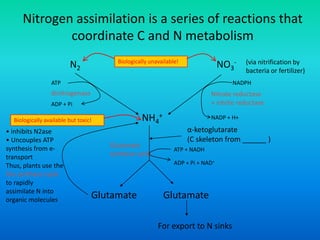
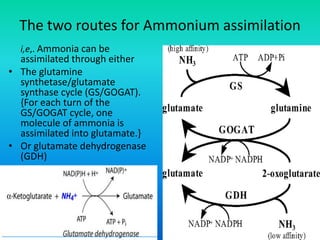
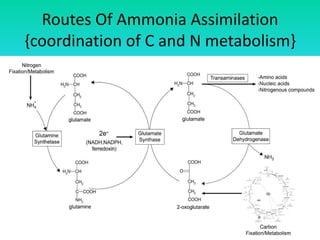
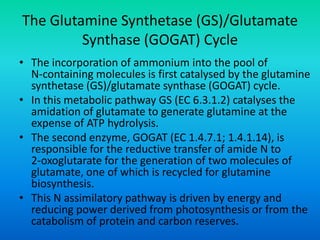
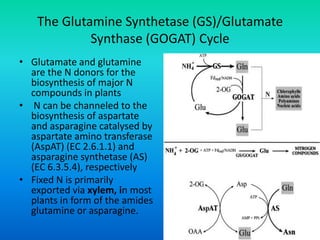
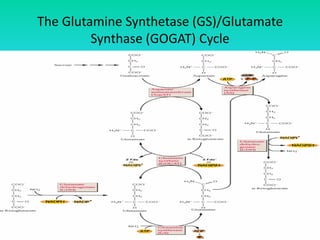
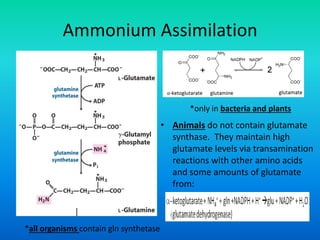
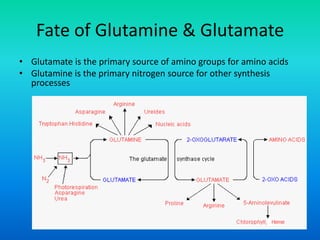
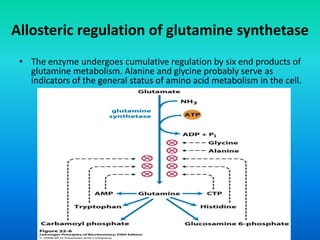
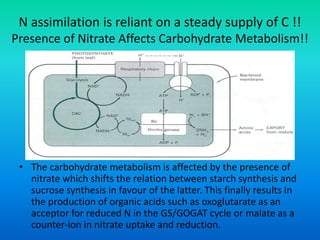
The document discusses nitrogen metabolism in plants, focusing on ammonium assimilation and the pathways for incorporating ammonia into organic compounds. It describes two main routes for ammonia assimilation involving glutamine synthetase and glutamate dehydrogenase, detailing the enzymatic processes required for nitrogen incorporation and the energy dynamics involved. Furthermore, it highlights the importance of nitrogen sources in biosynthesis and the regulation of glutamine synthetase by various metabolites.