Embed presentation
Download as PPSX, PPTX
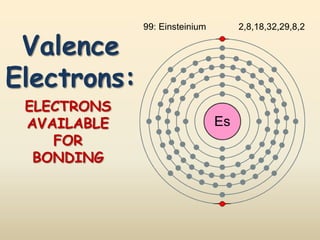


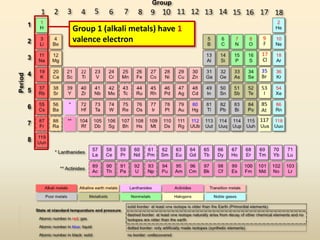
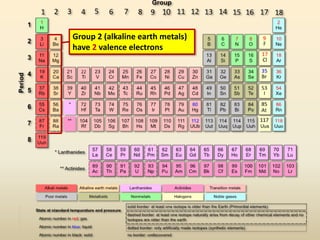
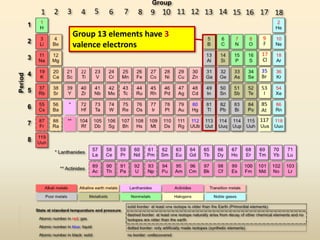
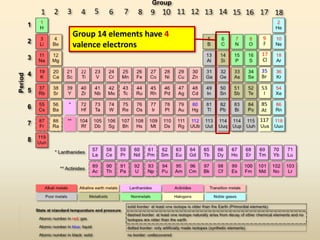
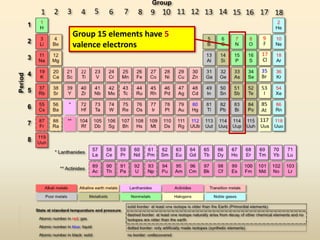
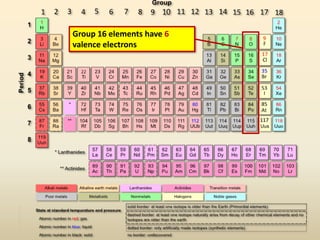
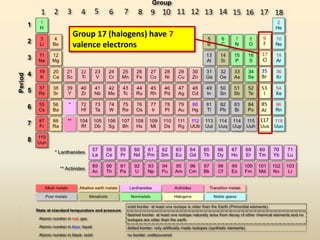
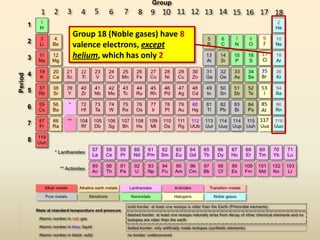
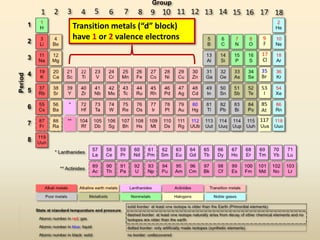
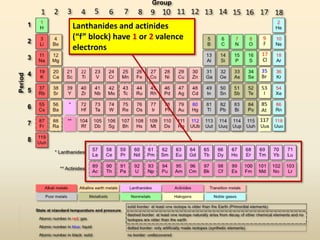
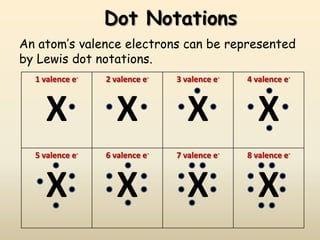
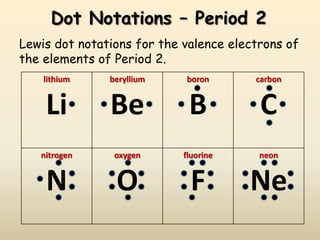
This document discusses valence electrons and how to determine the number available for bonding using the periodic table. It defines valence electrons as those in the outermost shell or energy level available for bonding. It provides examples of how many valence electrons elements in different groups have, from 1 to 8. It also introduces Lewis dot structures as a way to represent an atom's valence electrons and provides examples of Lewis dot structures for period 2 elements.














