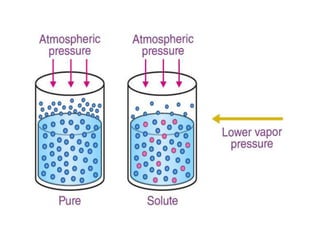
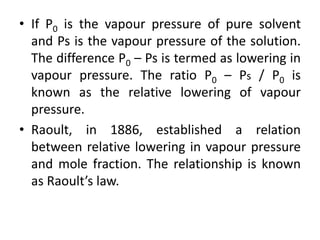
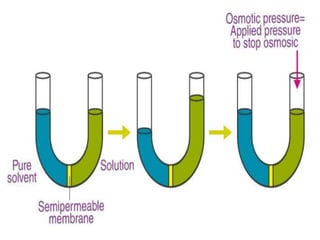
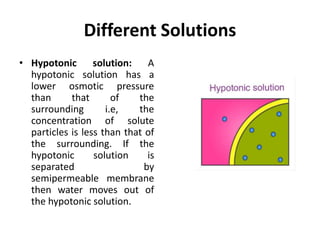
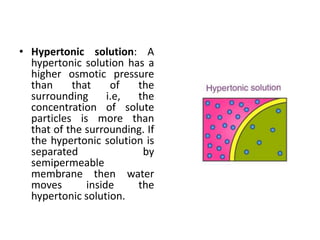
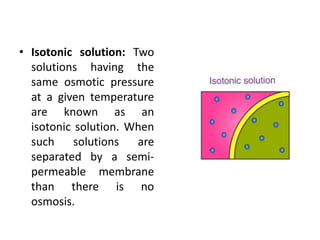
Colligative properties are properties of solutions that depend on the ratio of solute particles to solvent particles and not the chemical identity of the solute. The four main colligative properties are lowering of vapor pressure, elevation of boiling point, depression of freezing point, and osmotic pressure. These properties can be used to determine various solution characteristics like concentration but are more commonly used to understand phenomena like osmosis and melting ice on sidewalks for safety.