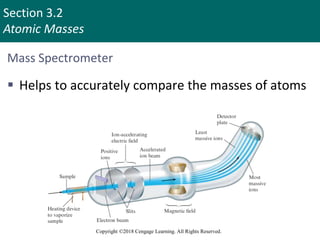
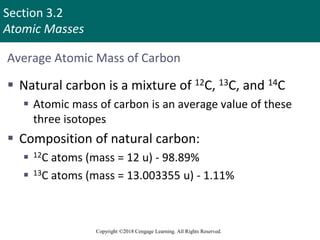
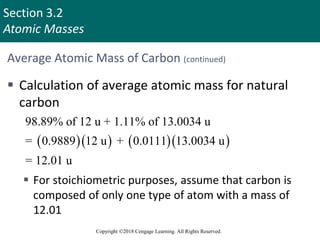
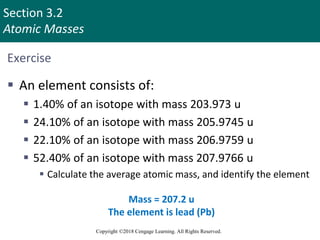
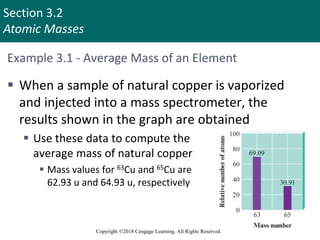
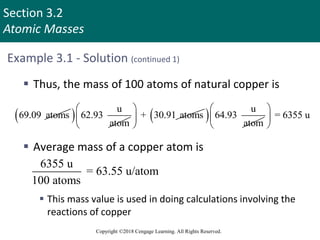
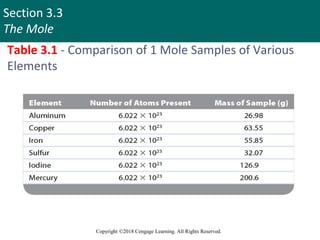
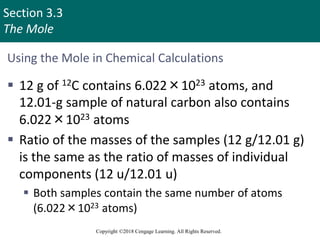
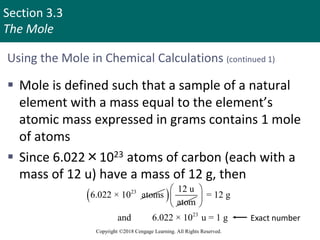
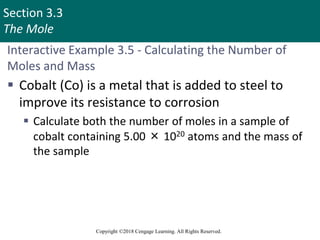
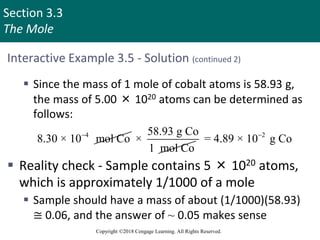
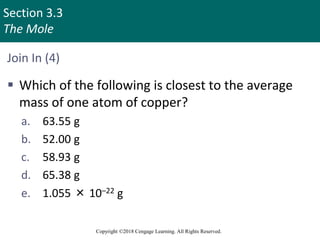
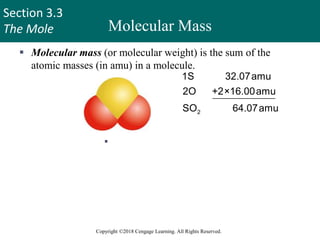
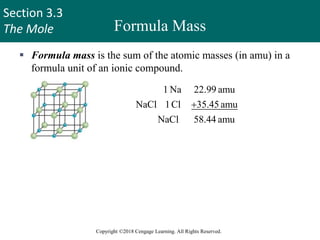
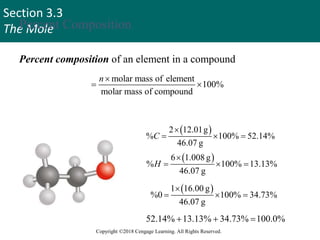
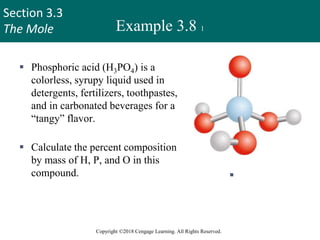
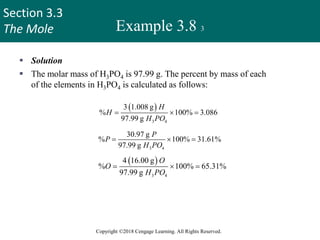
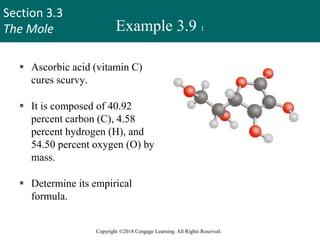
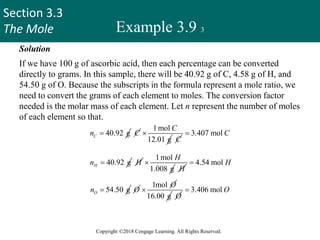
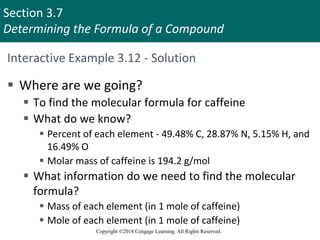
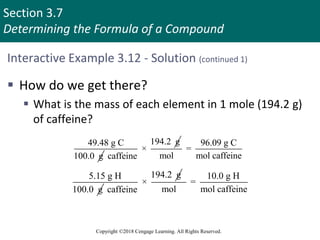
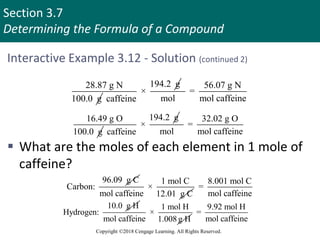
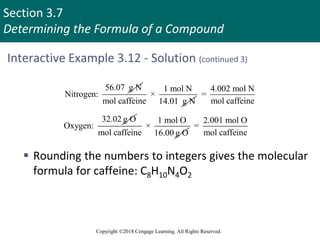
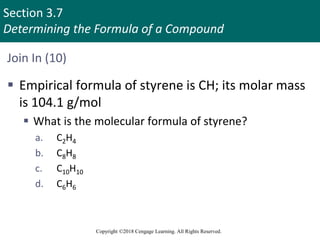
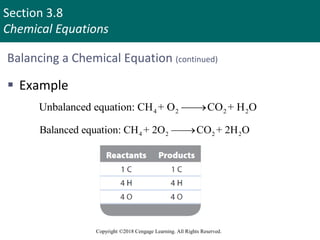
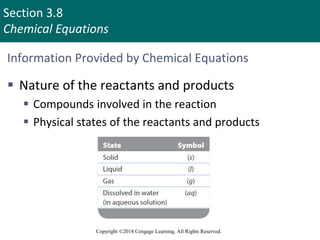
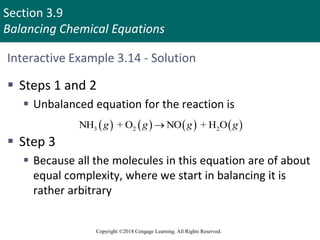
This document provides an overview of stoichiometry and the mole concept in chemistry. It discusses how counting by weighing can be used to determine the number of atoms in a sample based on its mass. The modern system of atomic masses uses carbon-12 as the standard, and mass spectrometry helps determine atomic masses accurately. Average atomic masses account for natural abundances of isotopes. The mole is defined as the amount of a substance with the same number of elementary entities (atoms, molecules, ions or other particles) as there are atoms in exactly 12 grams of carbon-12, and it allows direct conversion between mass and number of particles. Sample calculations are provided to demonstrate determining numbers of moles, atoms and masses in chemical problems.














![Section 3.3
The Mole
Copyright ©2018 Cengage Learning. All Rights Reserved.
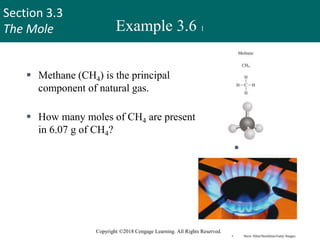
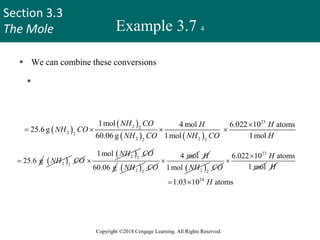
Example 3.7 1
How many hydrogen atoms
are present in 25.6 g of urea
[(NH2)2CO], which is used
as a fertilizer, in animal feed,
and in the manufacture of
polymers?
The molar mass of urea is
60.06 g.
urea
Ken Karp/McGraw-Hill 29](https://image.slidesharecdn.com/zumdahl10epptch03-231023185815-d2599dd4/85/Zumdahl10e_PPT_Ch03-pptx-29-320.jpg)










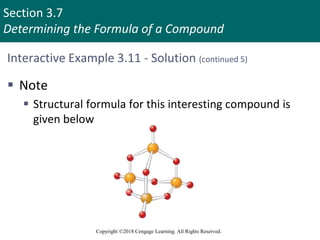
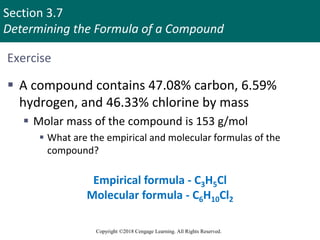















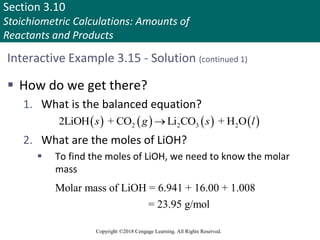
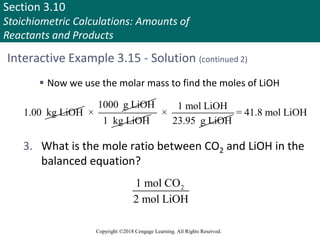
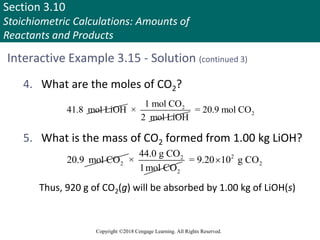

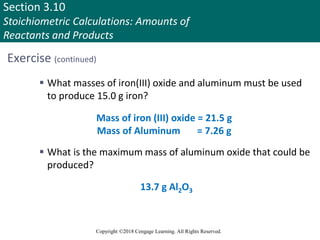
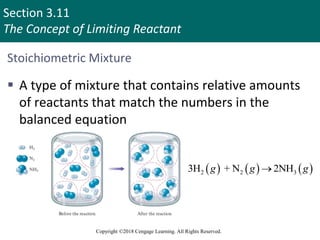


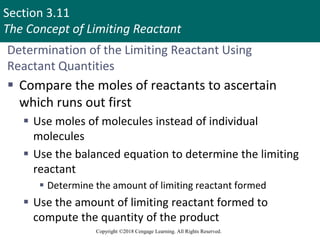




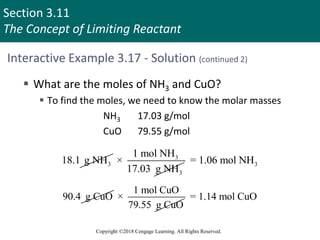

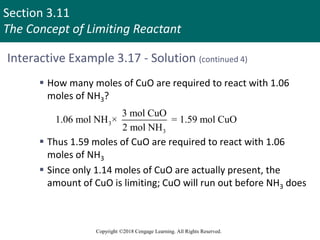
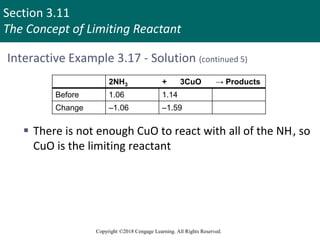
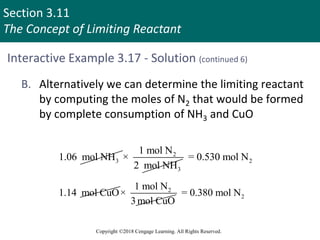

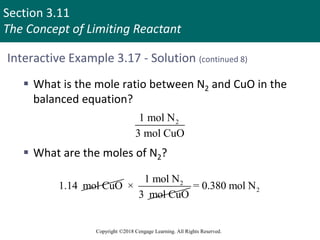
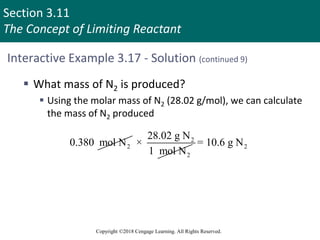







![Section 3.11
The Concept of Limiting Reactant
Copyright ©2018 Cengage Learning. All Rights Reserved.
Join In (15)
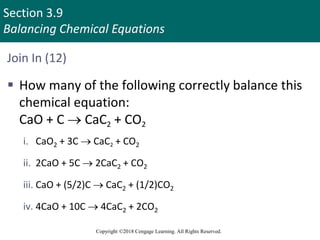
Limiting reactant in a reaction:
a. Has the smallest coefficient in a balanced equation
b. Is the reactant for which you have the fewest
number of moles
c. Has the lowest ratio of [moles available/coefficient
in the balanced equation]
d. Has the lowest ratio of [coefficient in the balanced
equation/moles available]
e. None of these](https://image.slidesharecdn.com/zumdahl10epptch03-231023185815-d2599dd4/85/Zumdahl10e_PPT_Ch03-pptx-128-320.jpg)



