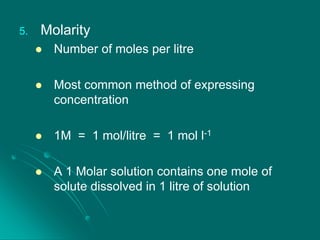
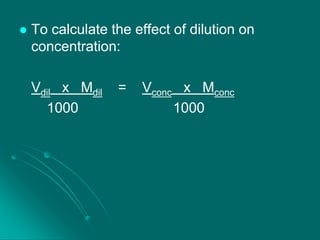
This document discusses different methods of expressing the concentration of solutions, including percentage by weight/weight (% w/w), percentage by weight/volume (% w/v), percentage by volume/volume (% v/v), parts per million (ppm), and molarity (M). It provides examples and practice problems for each method. It also covers how dilution affects concentration, standard solutions, primary standards including sodium chloride and potassium dichromate, and common substances that are not primary standards like sulfuric acid and sodium hydroxide.