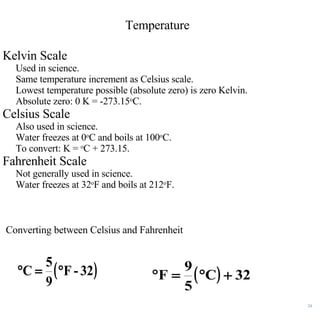
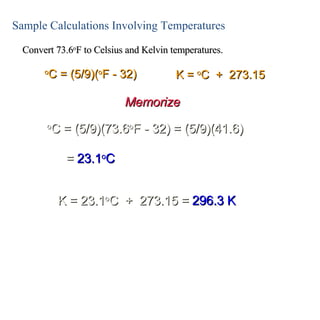
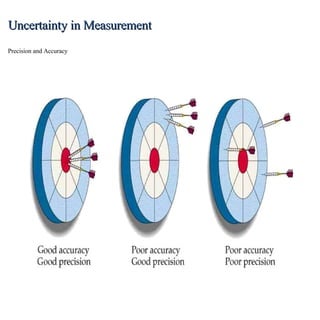
This lecture discusses scientific measurements and units. It covers the metric system and SI units, dimensional analysis, unit conversions, and significant figures. Key points include:
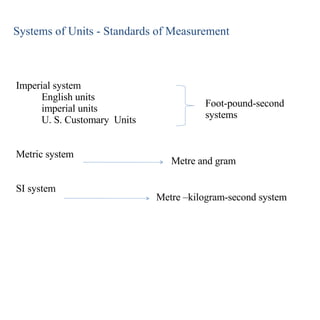
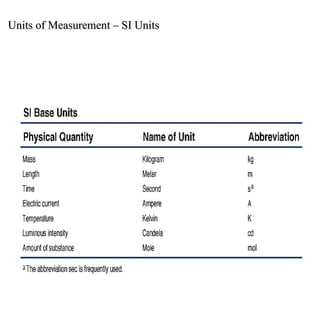
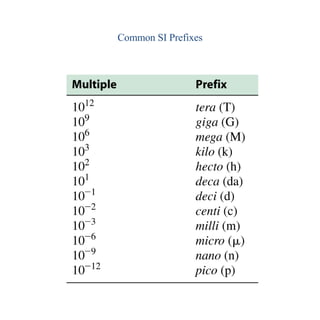
1. The metric system uses meters, grams, and seconds as fundamental units. There are seven base SI units including the meter for length and gram for mass.
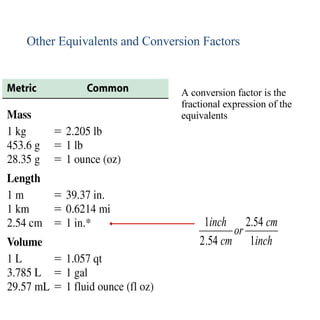
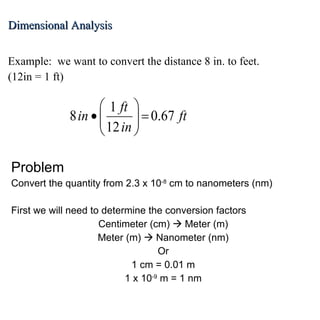
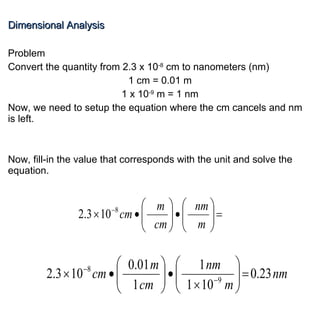
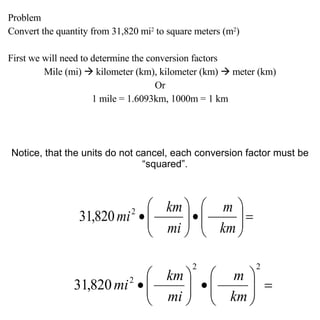
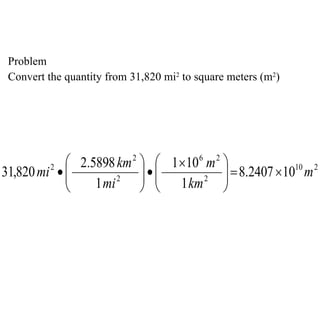
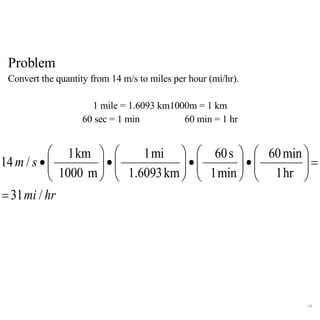
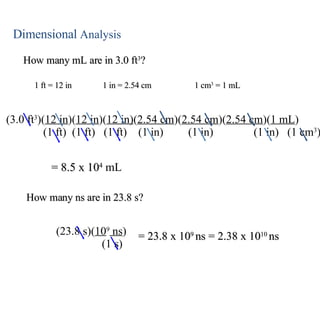
2. Dimensional analysis uses conversion factors to change between units while maintaining the correct dimensions. It is useful for solving chemistry problems.
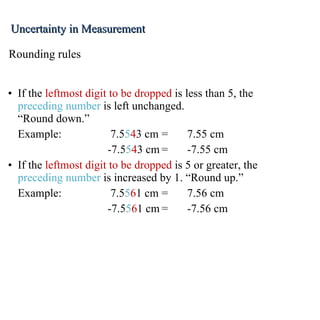
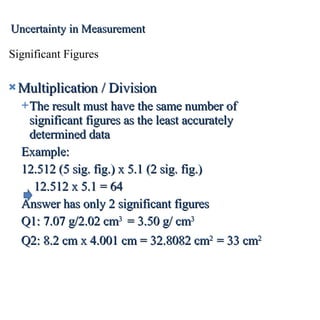
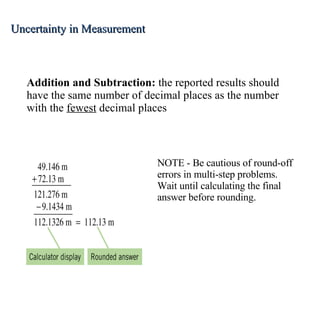
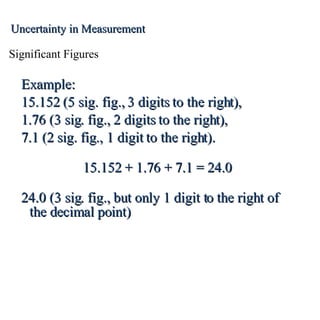
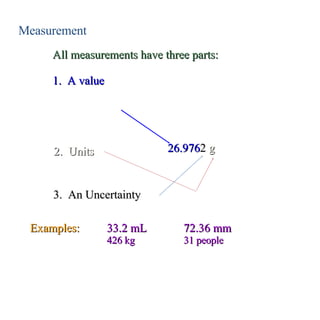
3. Significant figures indicate the precision of a measurement and how numbers should be rounded. Calculations are rounded according to whether they involve multiplication/division or addition/subtraction.










![Density Density - The mass of a unit volume of a material. density = mass/volume What is the density of a cubic block of wood that is 2.4 cm on each side and has a mass of 9.57 g? volume = [2.4 cm x 2.4 cm x 2.4 cm] density = (9.57 g)/(13. 8 cm 3 ) = 0.69 g/cm 3 = 0.69 g/mL Note that 1 cm 3 = 1 mL](https://image.slidesharecdn.com/lecture-34-1225792677349942-8/85/Lecture-3-4-22-320.jpg)