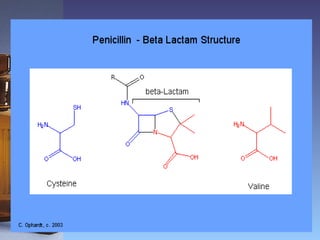
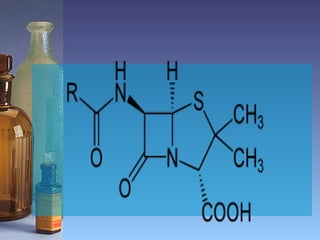
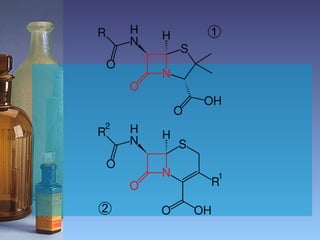
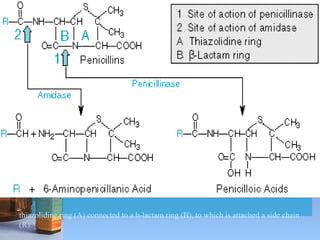
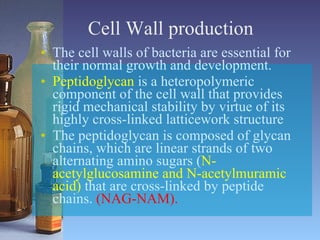
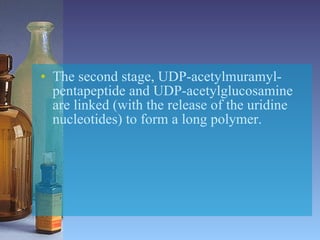
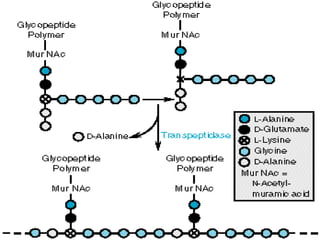
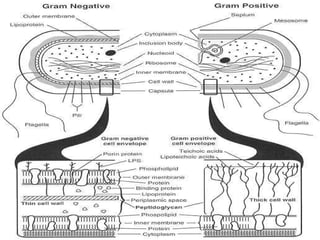
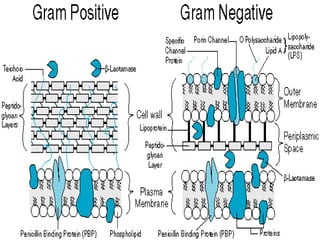
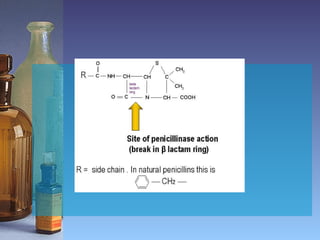
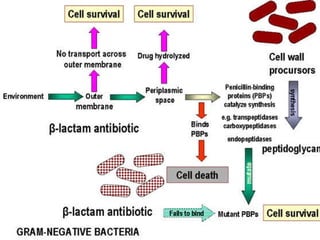
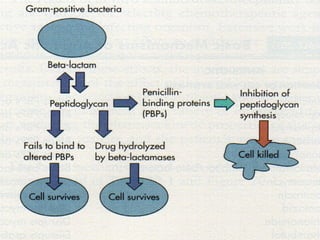
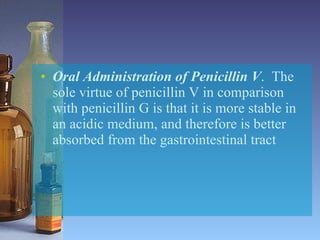
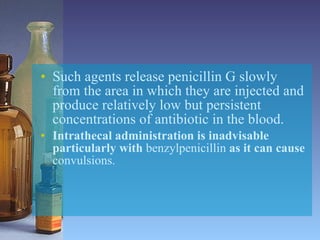
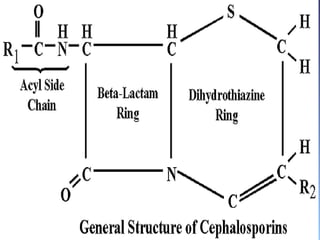
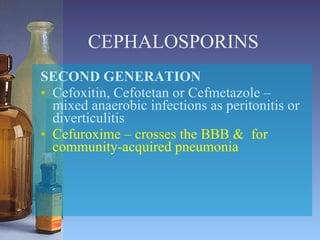
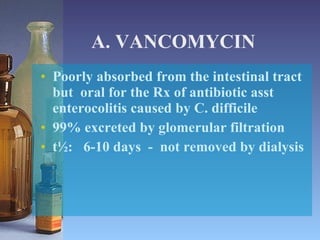
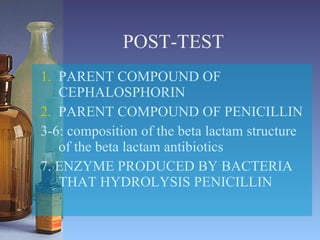
This document summarizes beta lactam antibiotics and other cell wall-active antibiotics. It discusses the history, structure, mechanisms of action, and classifications of penicillins. It provides details on specific penicillins including their spectra of activity, pharmacokinetics, dosages and adverse effects. The document covers key classes of beta lactam antibiotics including penicillins, cephalosporins, carbapenems, and other cell wall synthesis inhibitors.