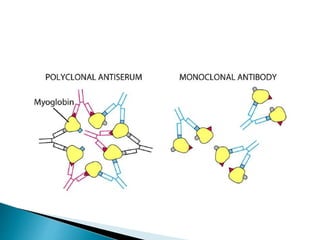
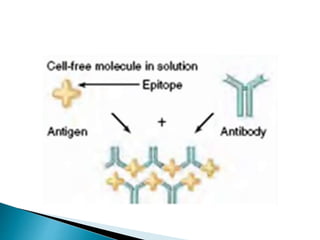
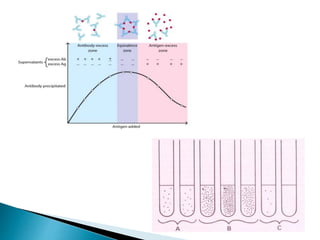
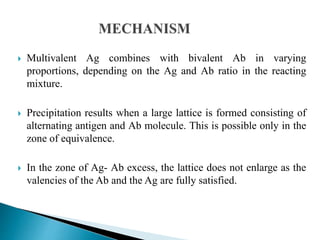
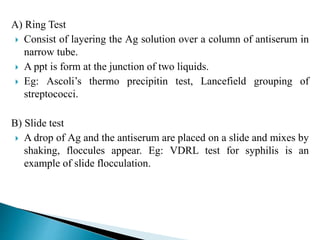
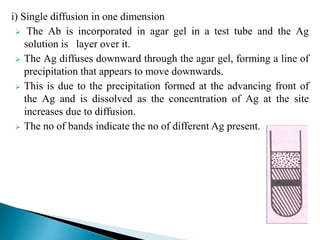
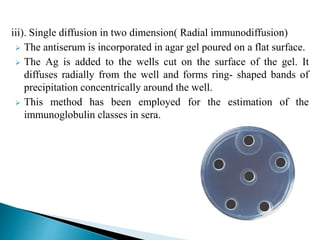
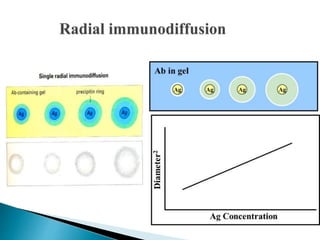
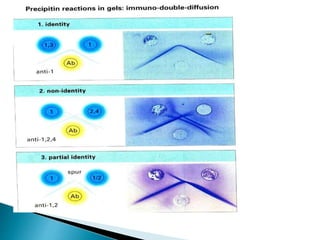
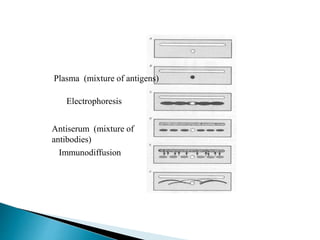
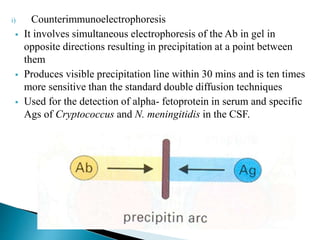
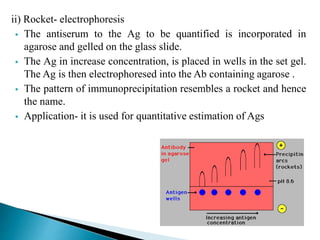
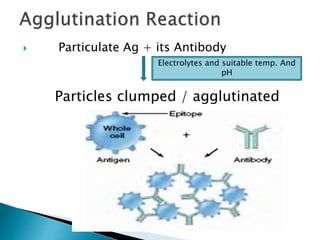
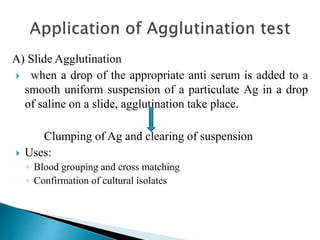
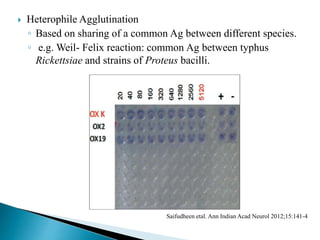
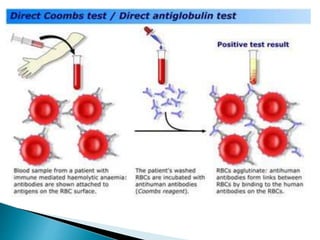
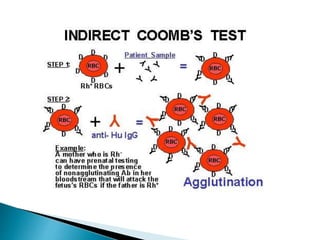
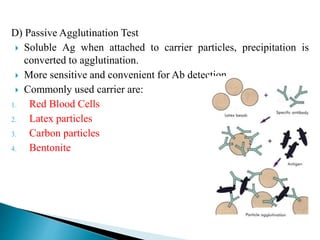
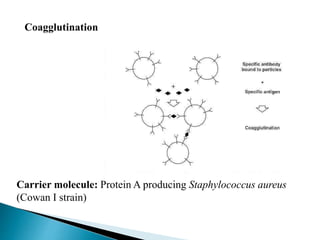
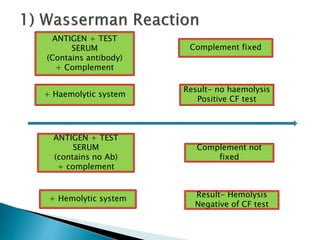
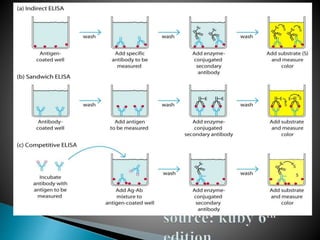
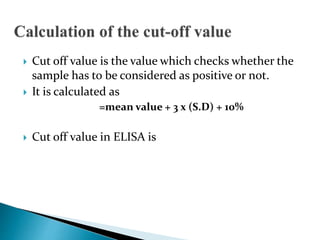
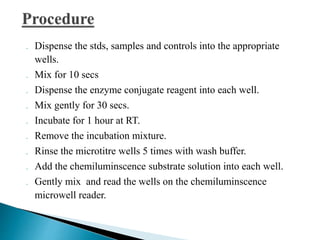
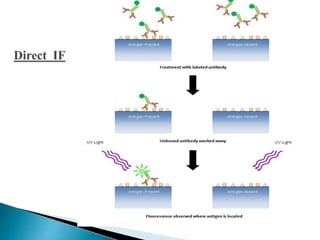
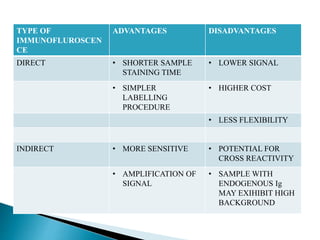
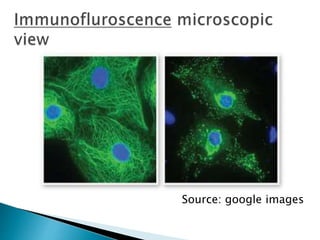
The document summarizes various immunological techniques including precipitation, agglutination, complement fixation, ELISA, CLIA, and fluorescence microscopy. Precipitation occurs when an antigen and antibody form an insoluble complex. It is influenced by temperature, pH, and antigen-antibody ratios. Agglutination involves cross-linking of particulate antigens by antibodies. Complement fixation tests utilize the ability of antigen-antibody complexes to activate the complement system. ELISA and CLIA are sensitive immunoassays that detect antigens or antibodies using enzyme conjugates and color or chemiluminescent substrates. Fluorescence microscopy uses fluorescent dye-labeled antibodies to visualize antigen localization.