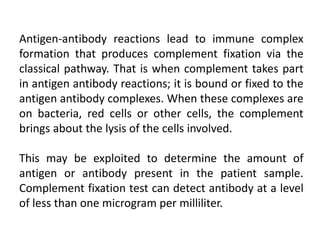
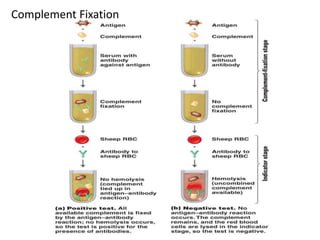
Complement fixation tests (CFT) detect antibodies that do not agglutinate or precipitate by measuring their ability to fix complement. CFT involves incubating patient serum with antigen and complement, then determining if complement is still available to lyse indicator cells. If complement is fixed in the antigen-antibody complex, it cannot lyse the indicator cells, indicating antibody presence. CFT can detect antibody levels below 1 microgram/mL, but it is time-consuming and not sensitive enough for immunity screening due to occasional nonspecific reactions. Interpretation involves whether indicator cell lysis occurs, indicating the absence or presence of antibodies in the patient serum.