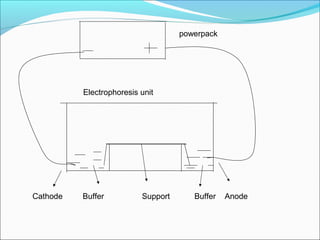
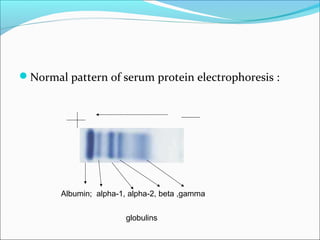
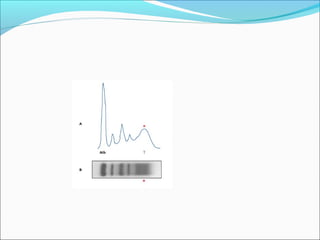
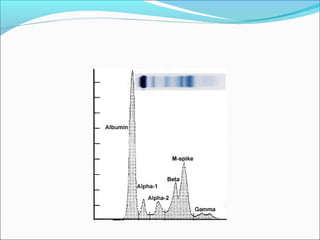
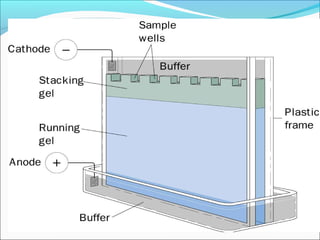
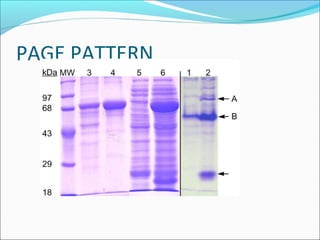
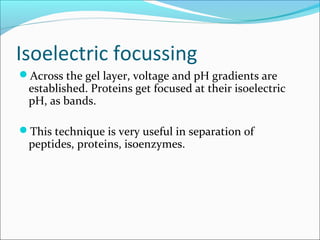
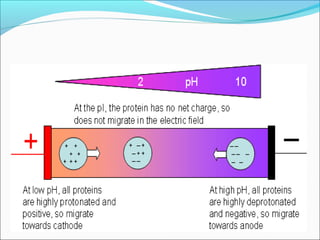
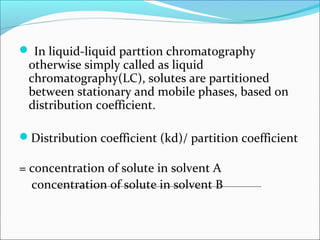
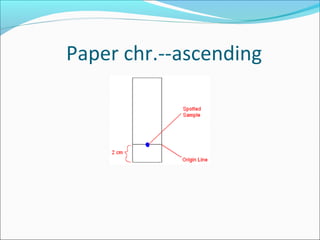
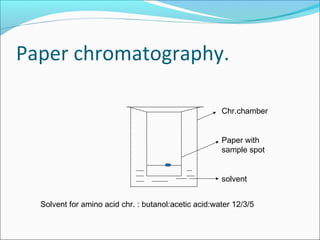
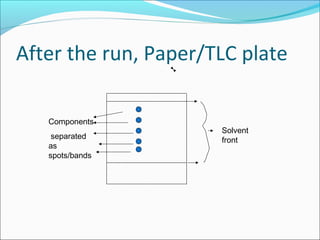
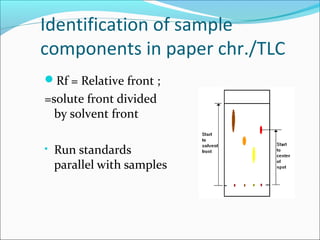
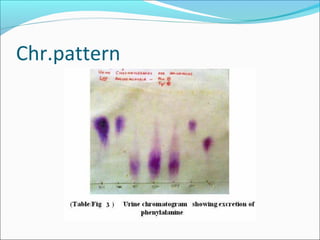
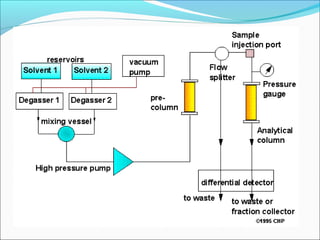
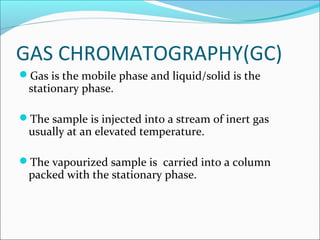
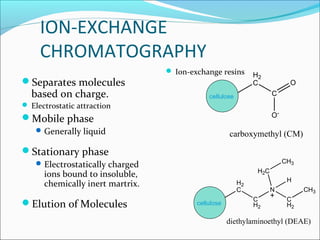
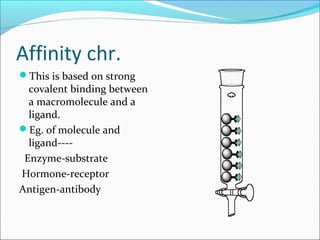
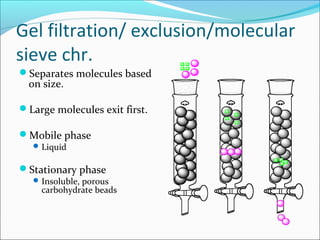
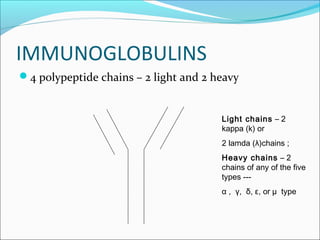
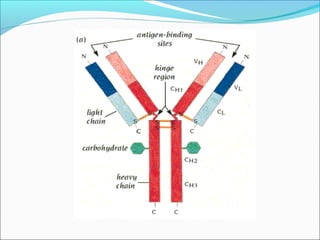
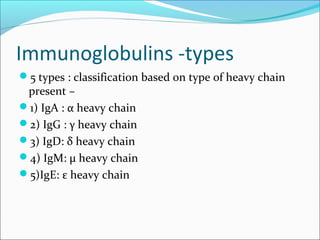
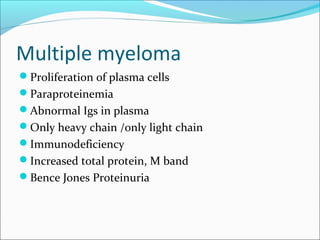
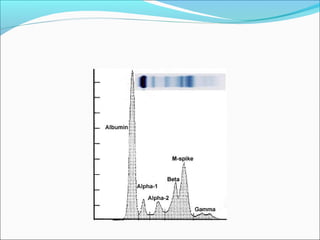
Electrophoresis is a technique used to separate charged biomolecules like proteins and nucleic acids. It works by applying an electric field to migrate these molecules through a buffer solution or gel based on their charge to mass ratio. The first major use of electrophoresis was in 1937. Key aspects include using a buffer to maintain pH and conductivity, as well as staining and destaining techniques to visualize separated biomolecules. Common types include gel electrophoresis using agarose or polyacrylamide gels as well as paper electrophoresis. Applications include clinical diagnosis and protein research.