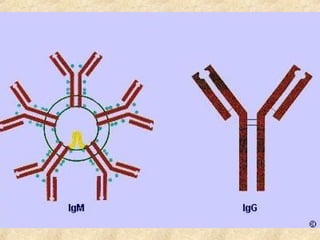
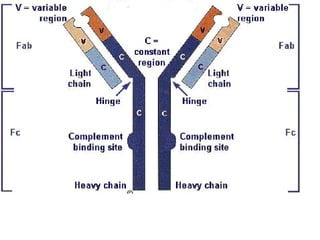
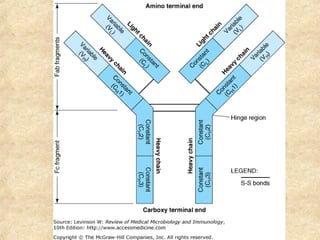
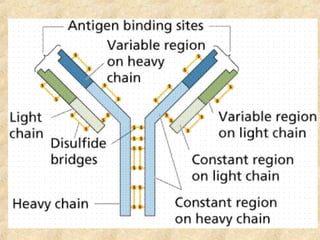
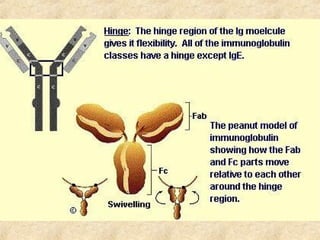
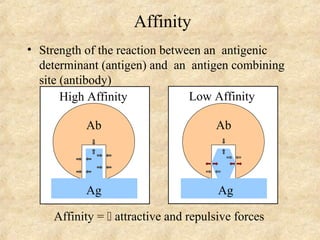
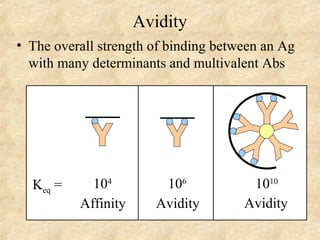
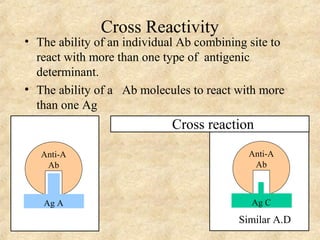
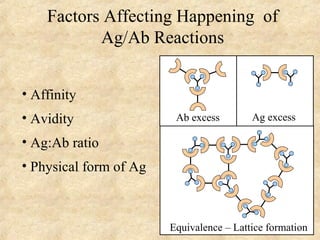
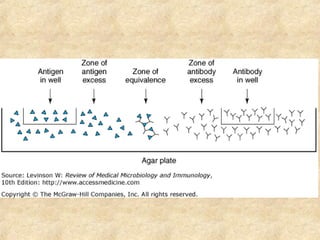
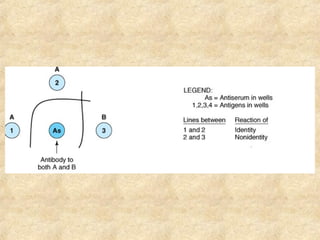
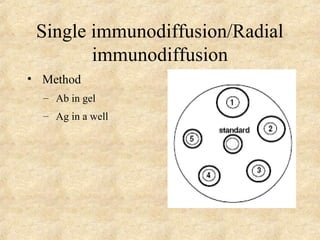
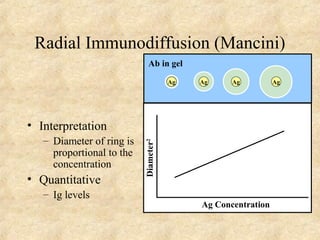
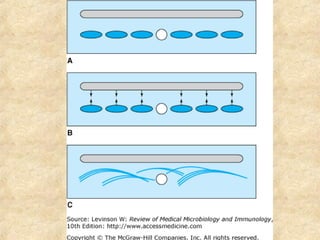
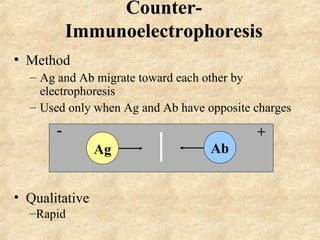
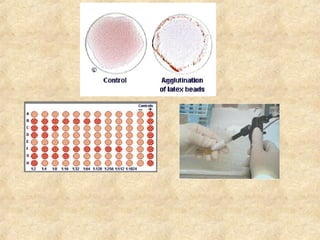
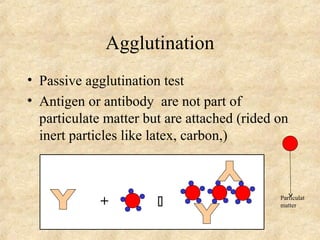
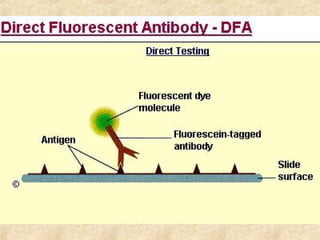
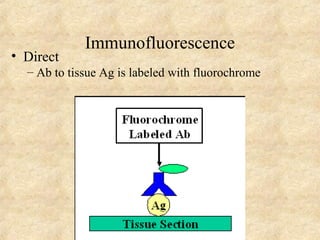
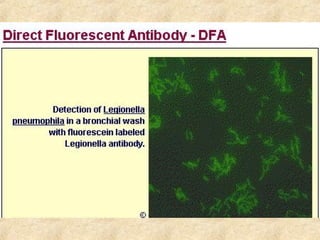
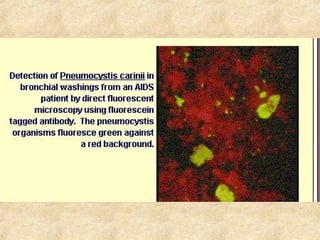
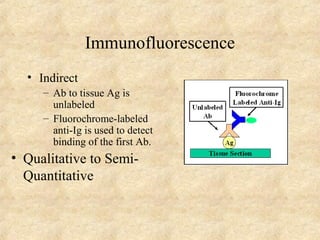
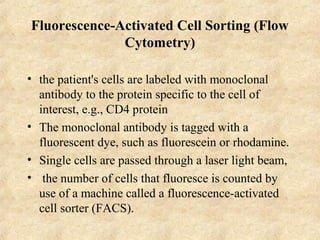
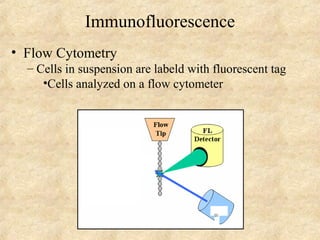
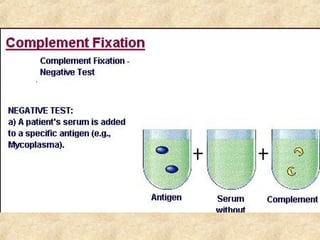
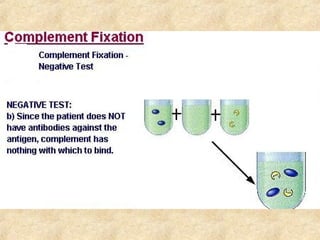
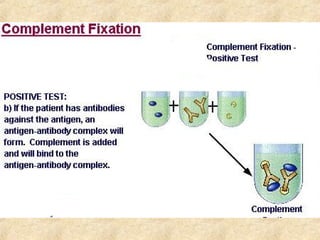
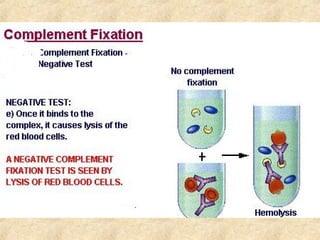
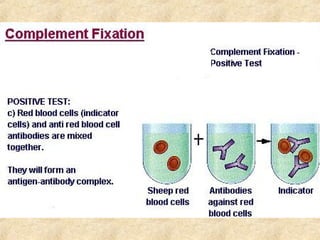
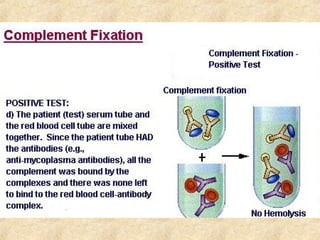
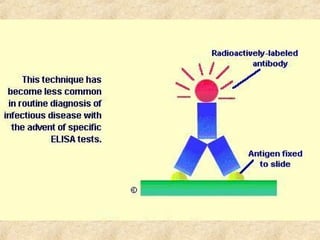
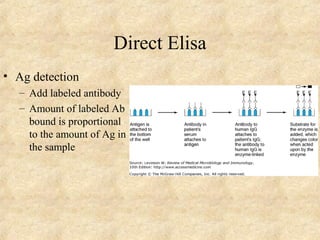
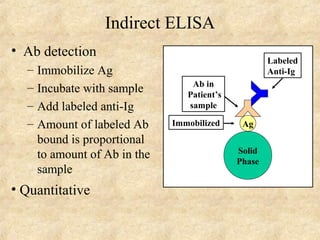
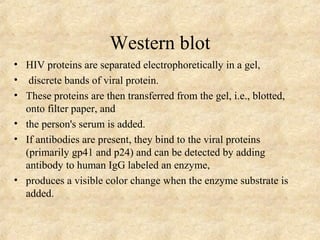
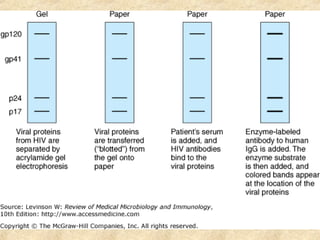
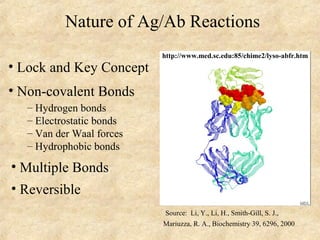
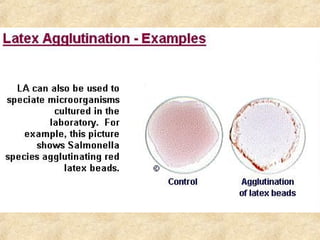
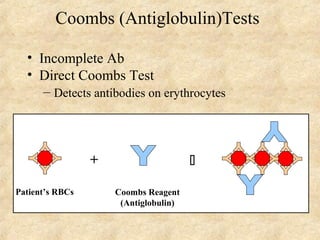
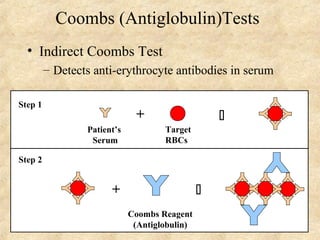
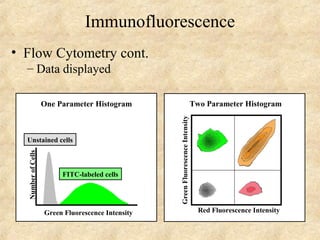
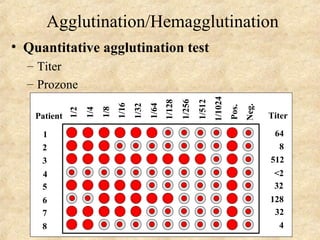
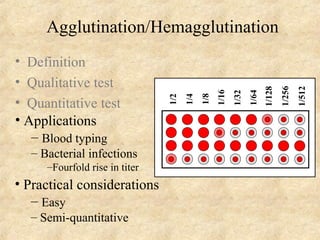
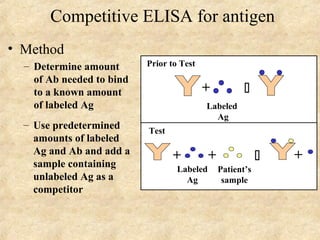
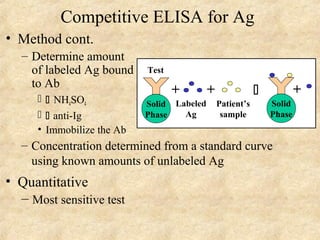
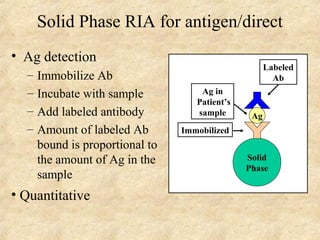
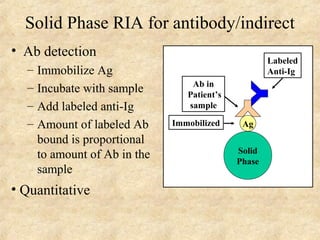
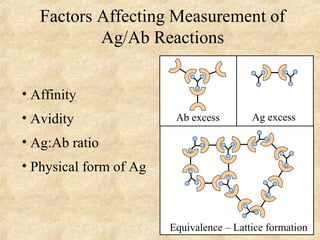
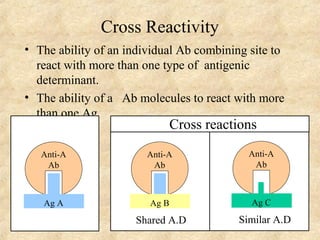
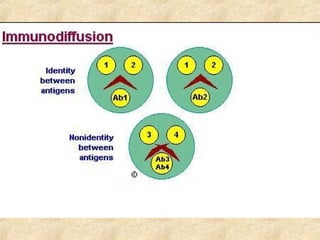
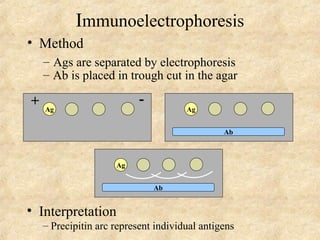
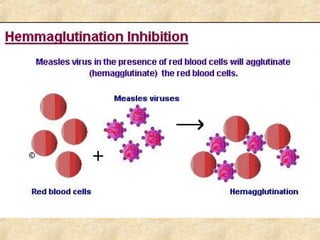
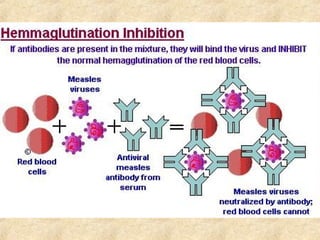
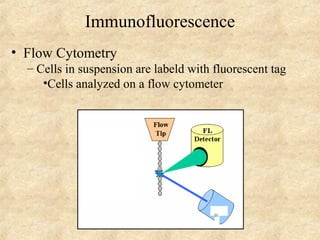
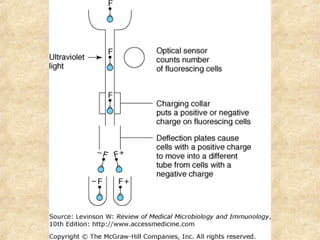
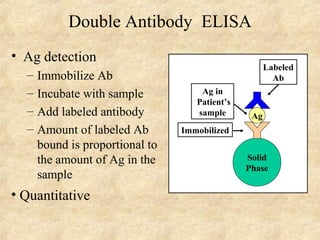
This document discusses antigen-antibody reactions and various tests used to detect antigens and antibodies. It describes the components of plasma including antibodies (IgG, IgA, IgM, IgE, IgD). It also discusses the functions of antibodies in neutralizing toxins/viruses, opsonization, complement activation, and preventing microbial attachment. Various factors that affect antigen-antibody reactions like affinity, avidity, antigen-antibody ratio are also described. Finally, it summarizes different types of tests used to detect antigens and antibodies including precipitation tests, agglutination, ELISA, immunofluorescence, complement fixation, radioimmunoassay, and neutralization tests.