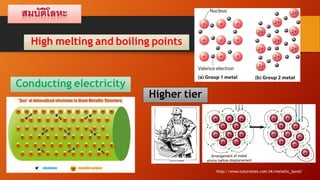
Metallic bonding is the strong attraction between closely packed positive metal ions and delocalized electrons. This bonding gives metals high melting and boiling points. Metals are good conductors of heat and electricity due to their delocalized electrons, which are free to move and transfer energy. Their dense, closely packed atomic structure results in high density as well as malleability and ductility, as the layers of atoms can easily slide past one another.