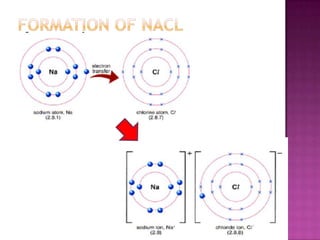
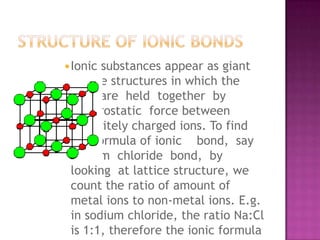
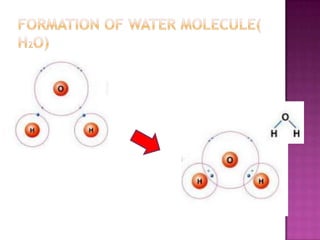
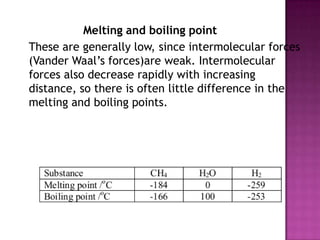
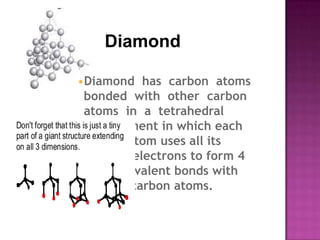
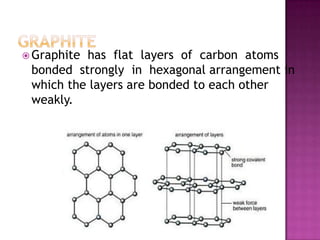
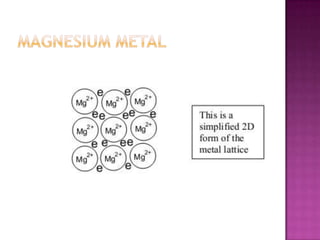
The document discusses different types of bonding: ionic, covalent, and metallic. Ionic bonding involves the transfer of electrons between metals and non-metals to form ions. Covalent bonding involves the sharing of electron pairs between non-metals. Metallic bonding involves a sea of delocalized electrons that are attracted to cationic metal ions arranged in a lattice. Each type of bonding results in different material properties depending on whether electrons are transferred, shared, or free to move.