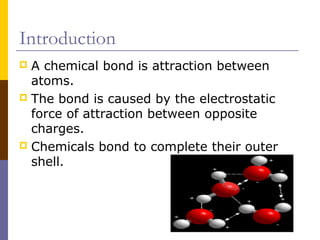
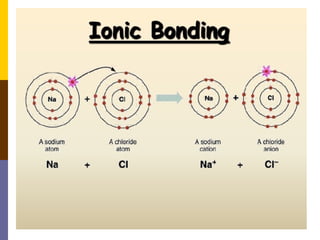
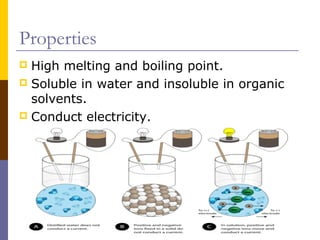
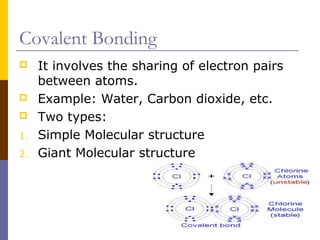
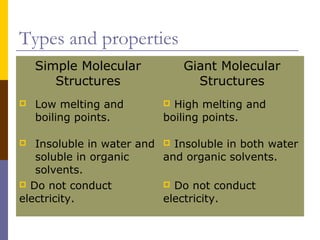
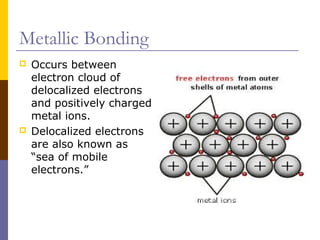
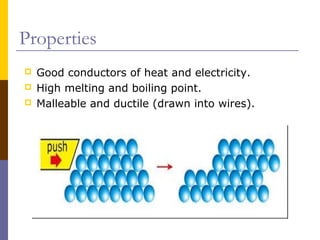
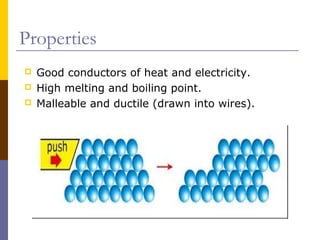
This document discusses different types of chemical bonding: ionic bonding which involves the transfer of electrons between metals and non-metals and results in charged atoms attracting each other; covalent bonding which involves the sharing of electron pairs between atoms and can result in simple molecular or giant molecular structures; and metallic bonding which occurs between delocalized electrons in metal ions and positively charged metal ions. Each type of bonding is described along with examples and properties such as conductivity, melting/boiling points, and solubility.