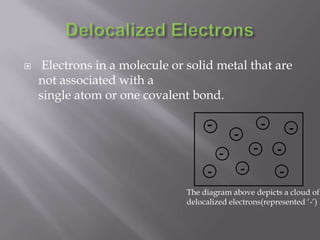
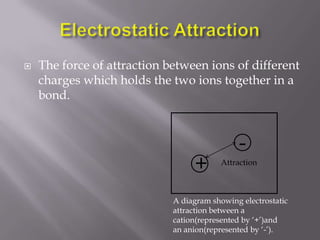
Metallic bonding occurs when metal atoms bond through delocalized electrons. Metals form a lattice structure with positive metal ions surrounded by a sea of delocalized electrons that are attracted to the positive ions. This bonding allows metals to have distinctive physical and chemical properties including malleability, conductivity, and high melting points. Metallic bonding is important for creating alloys and is found in many materials used in daily life.