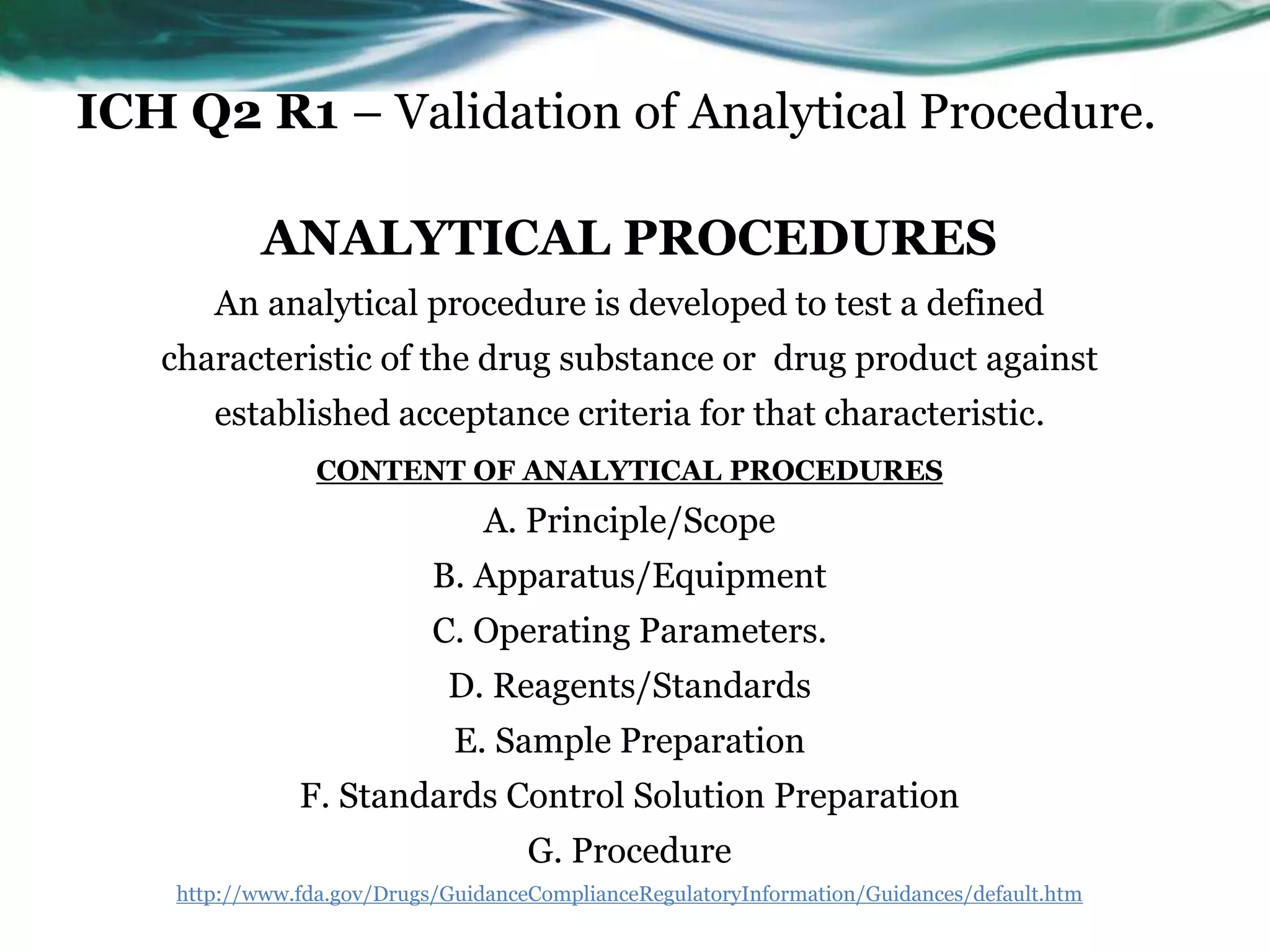
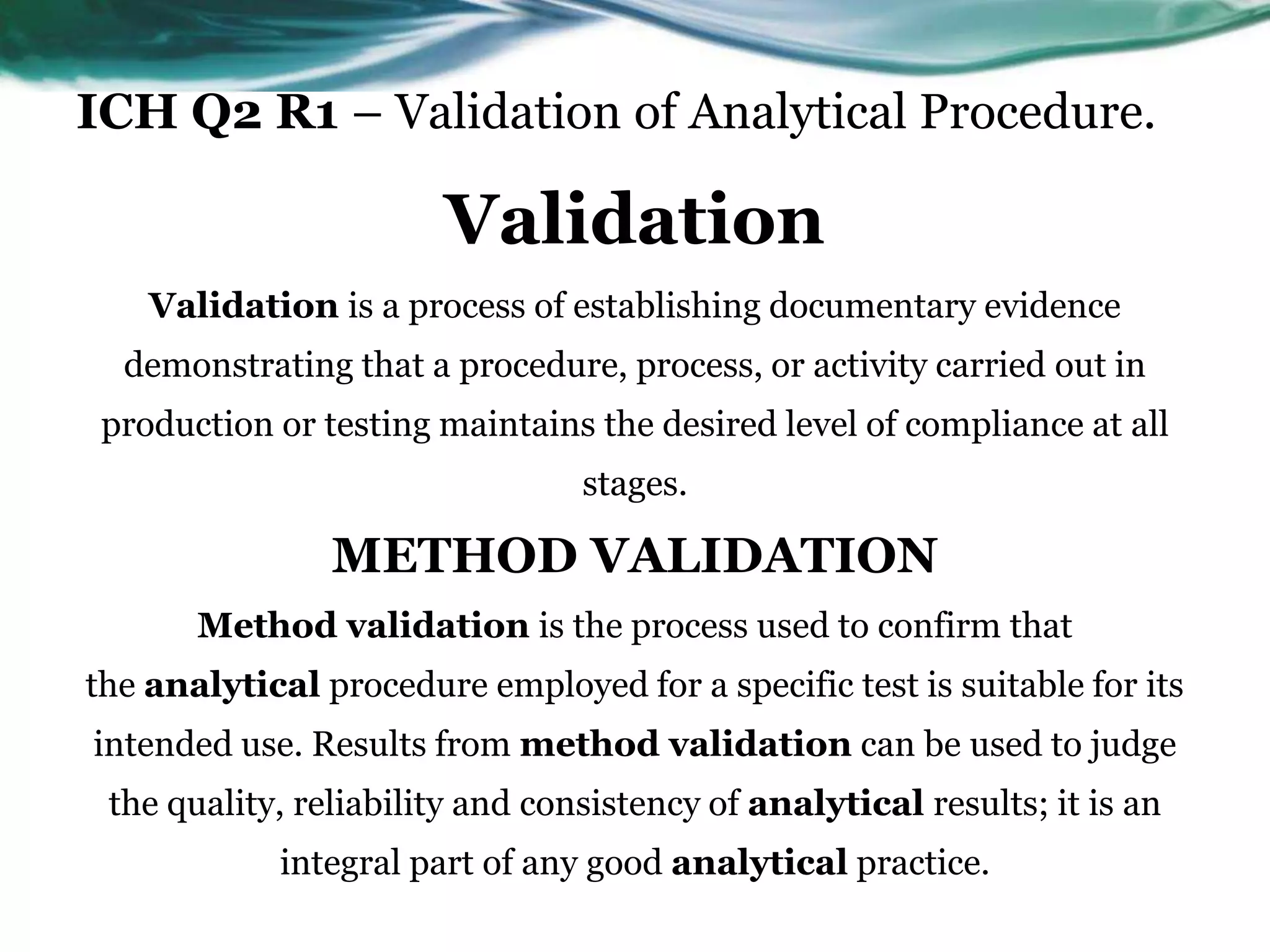
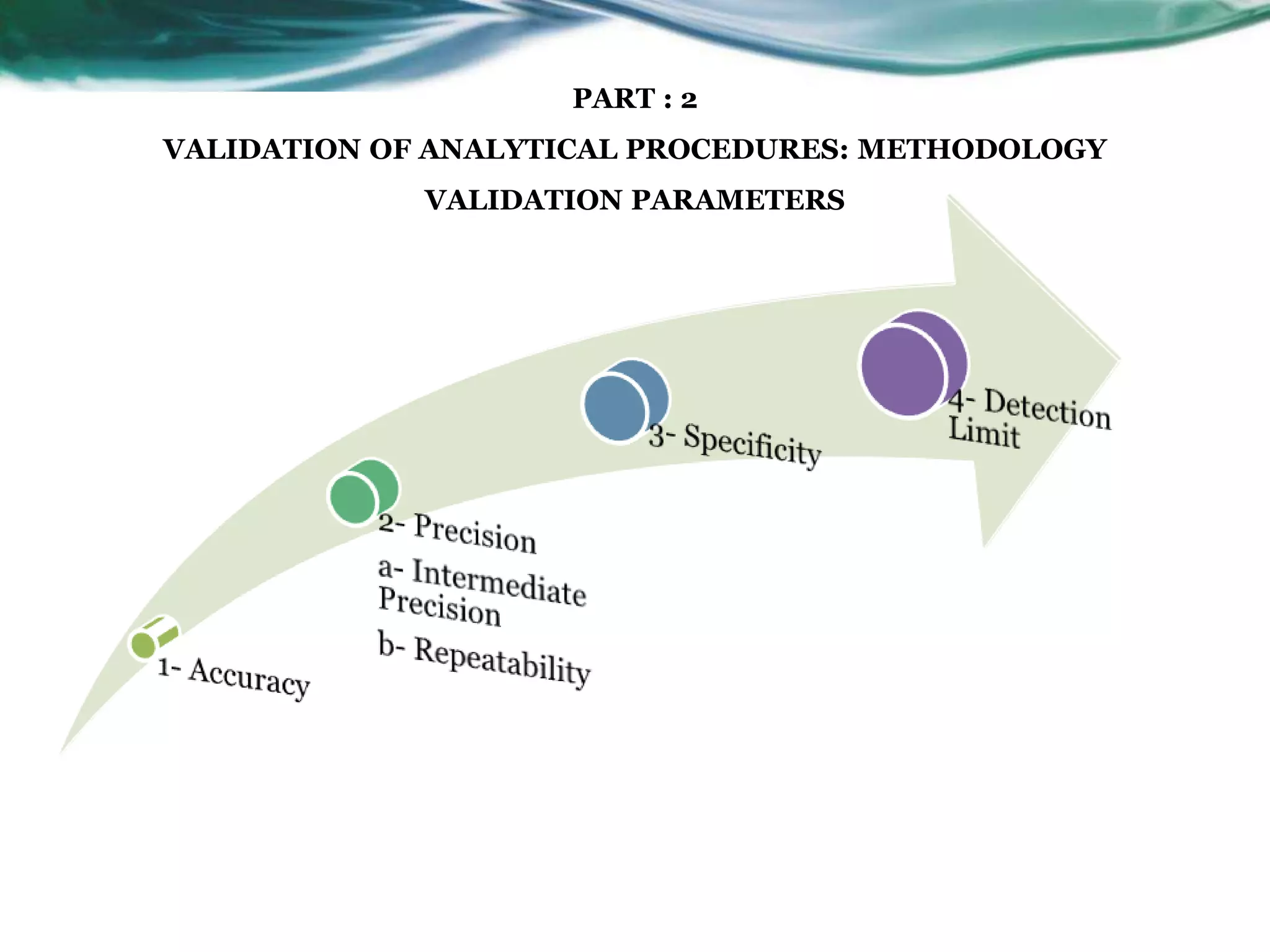
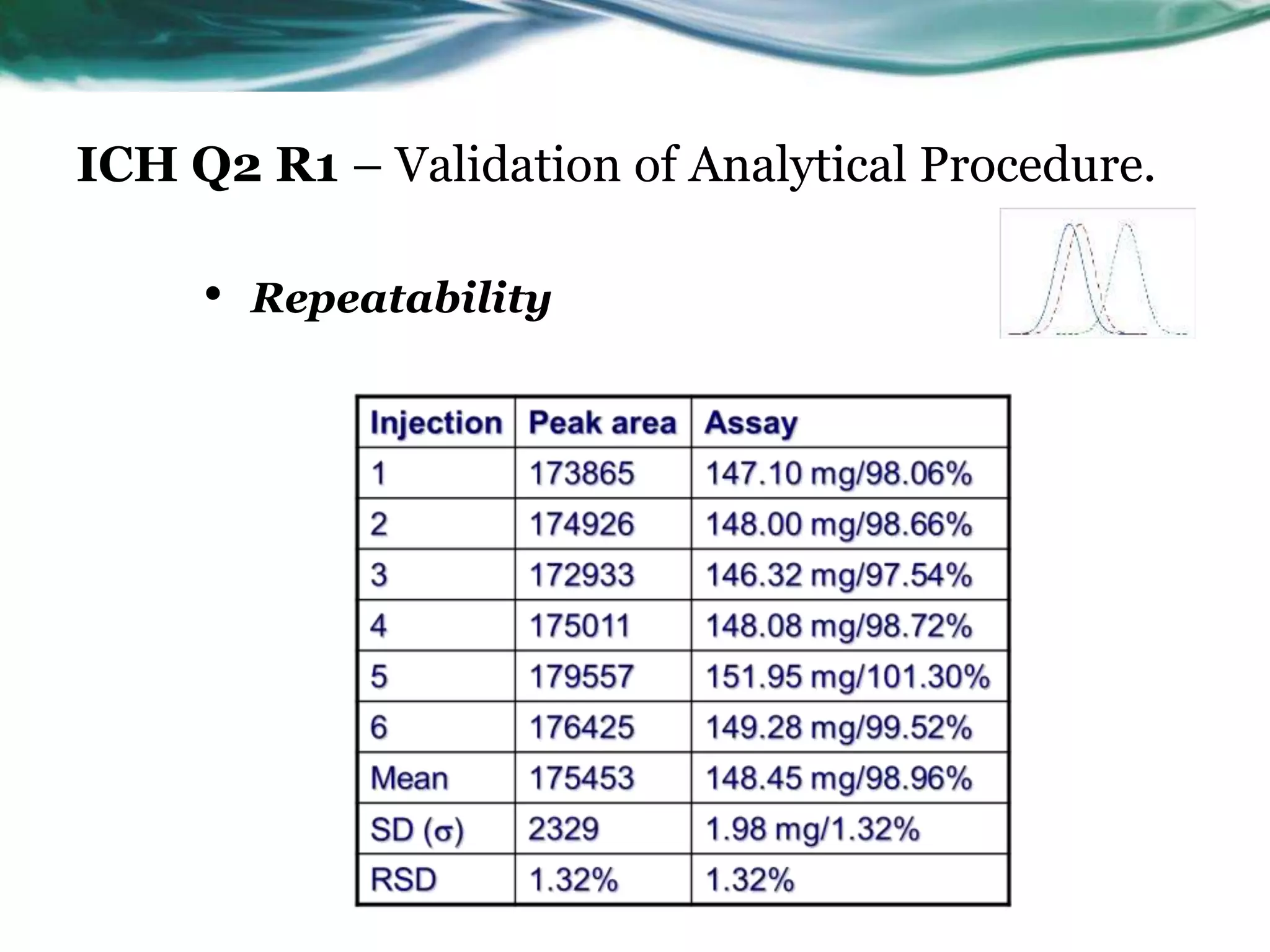
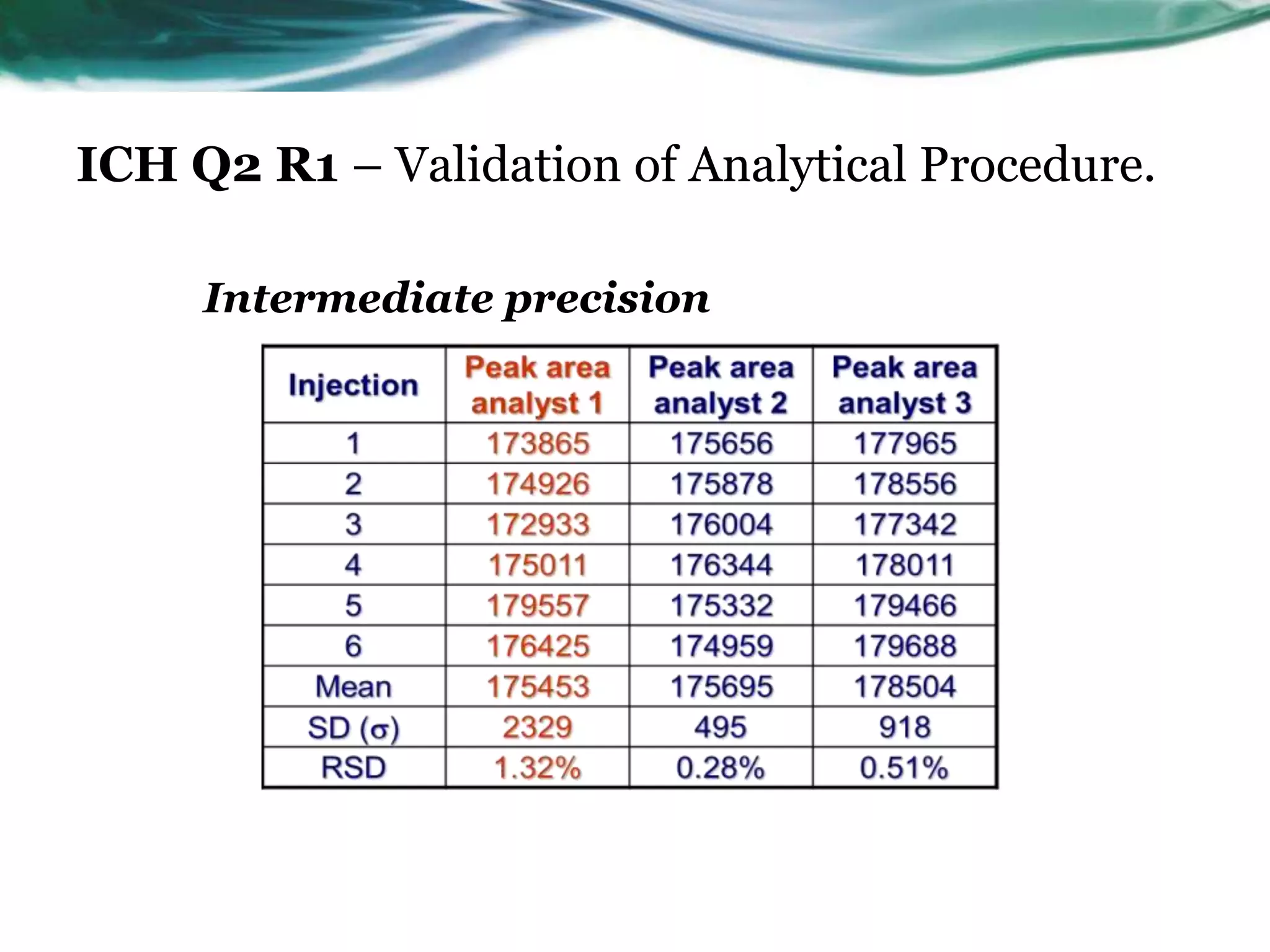
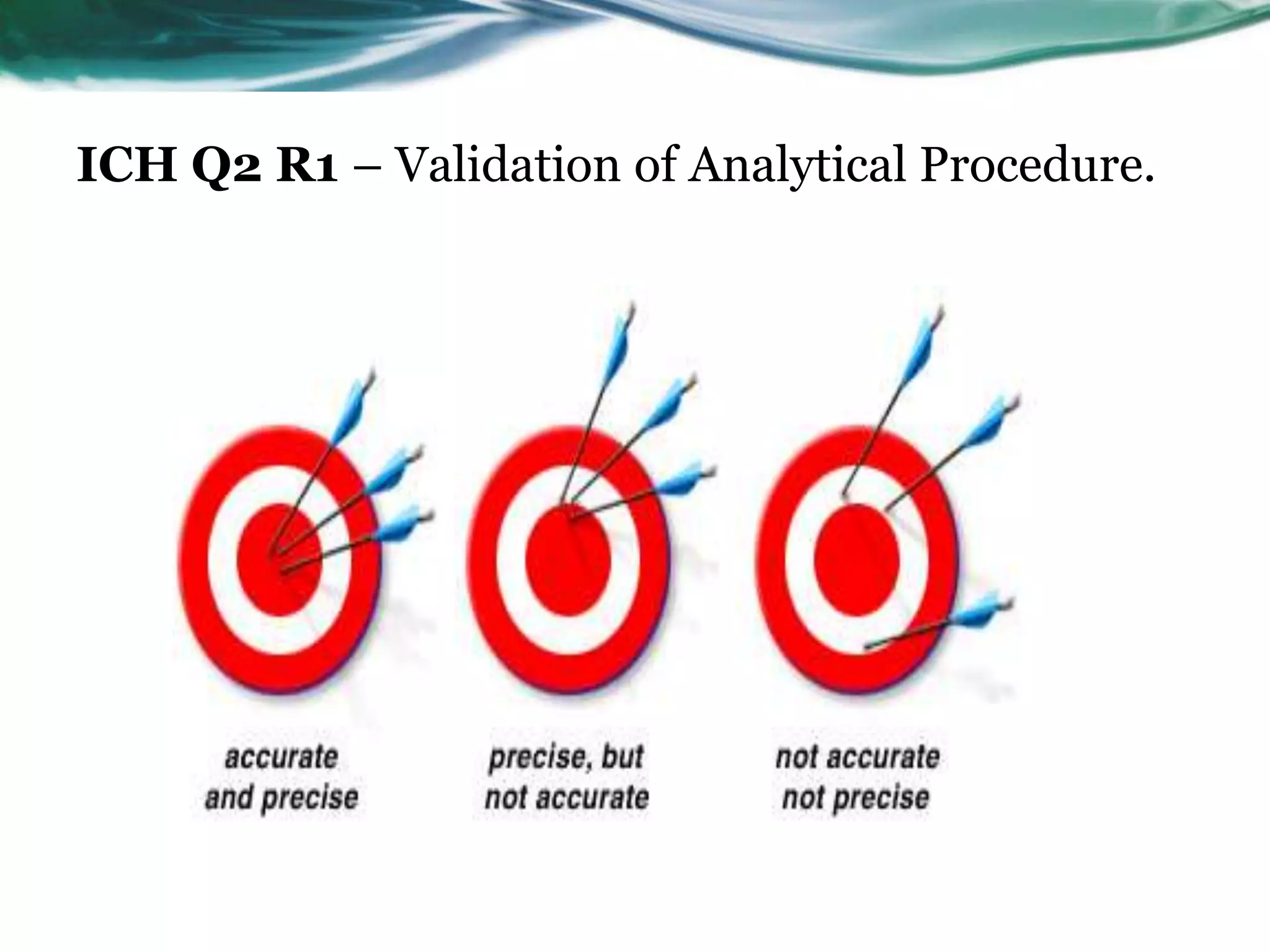
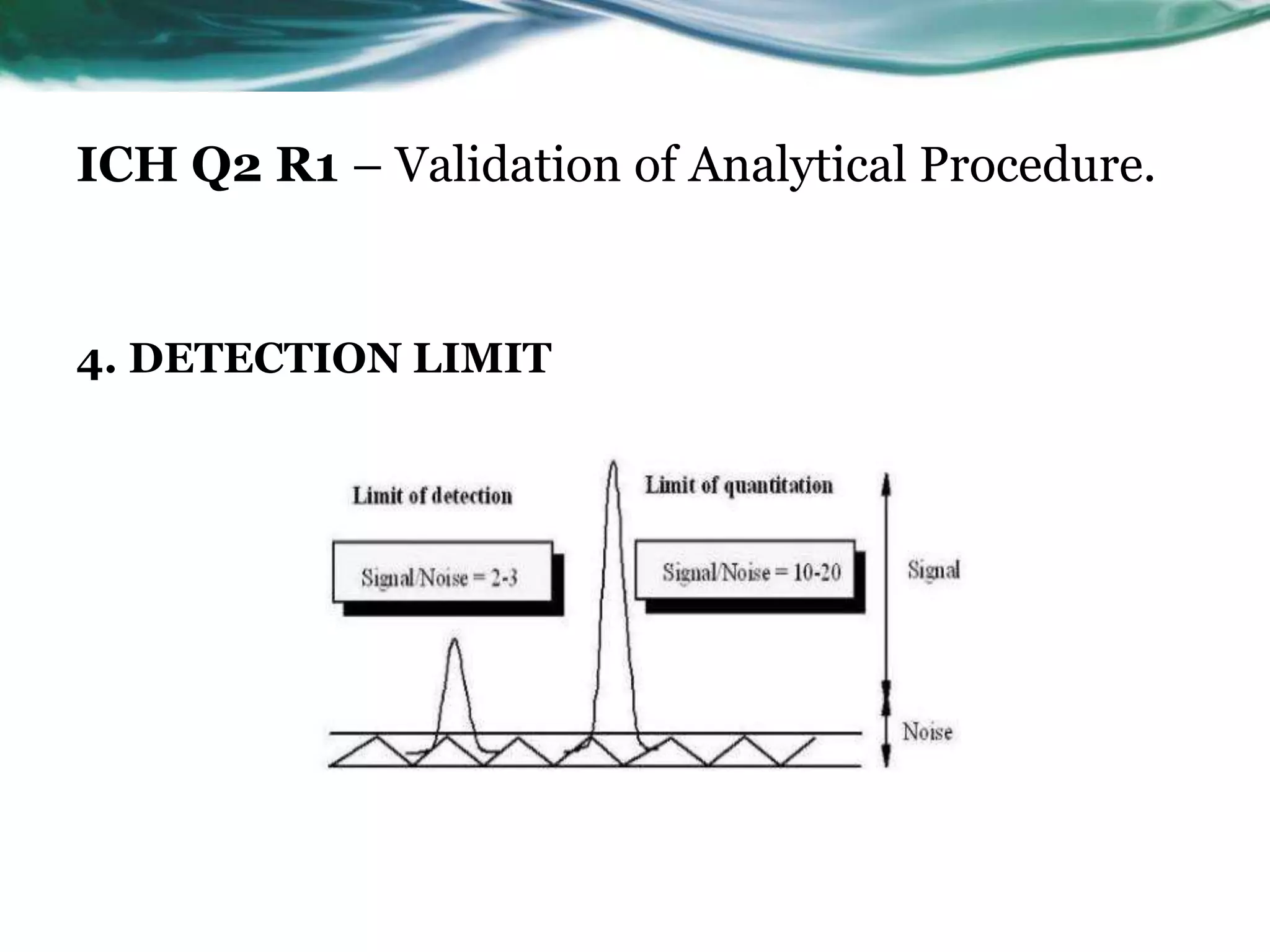
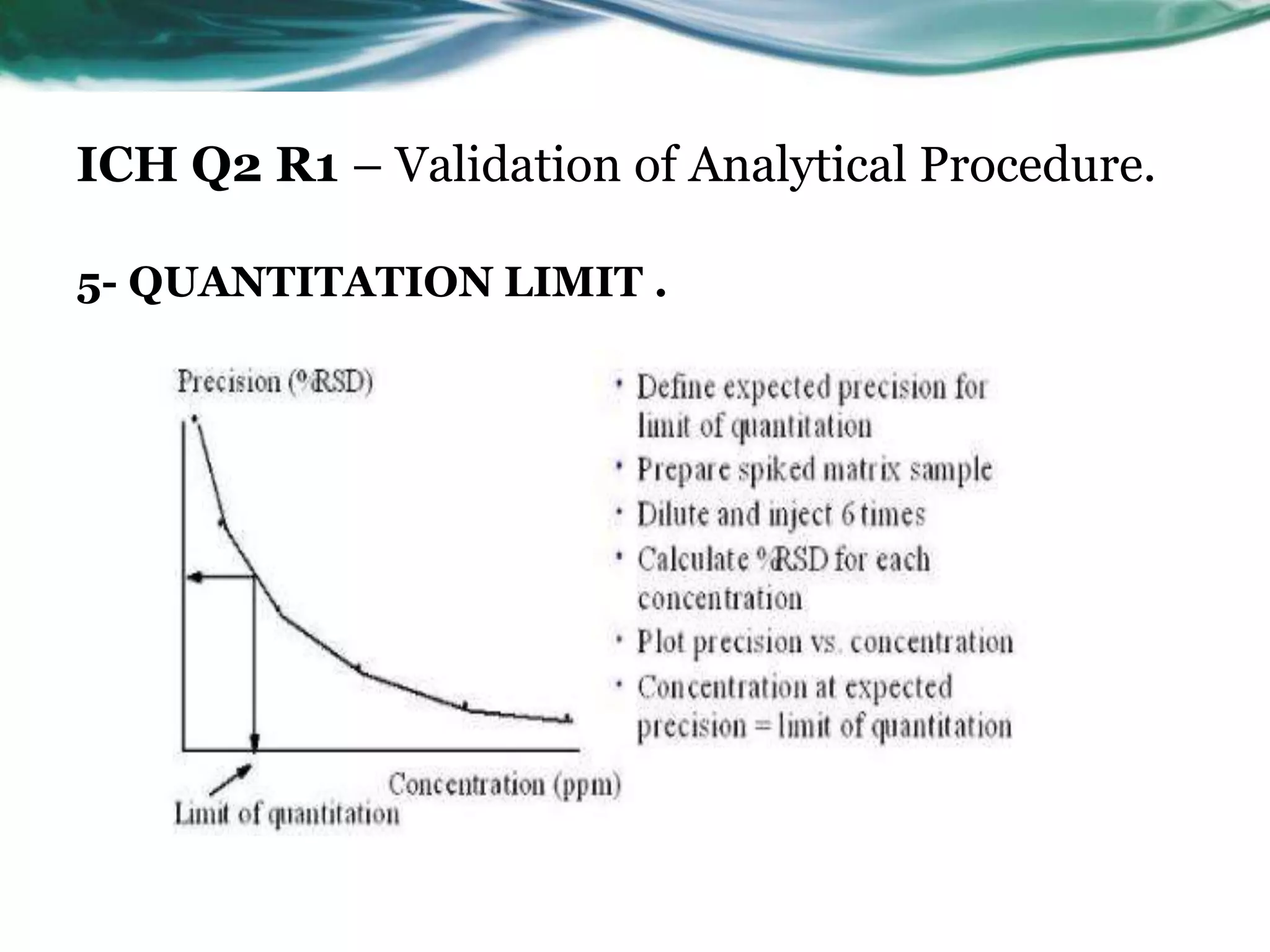
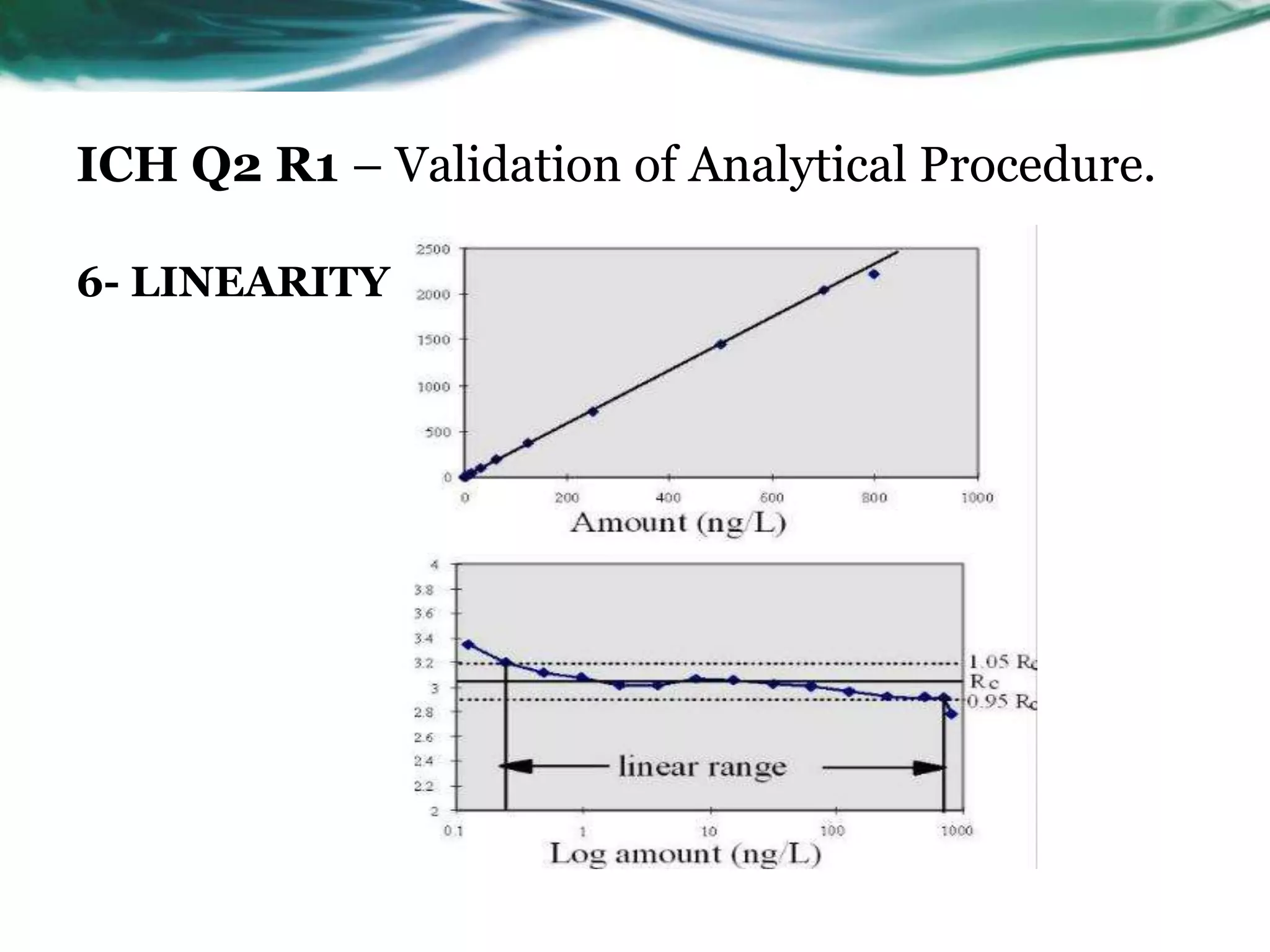
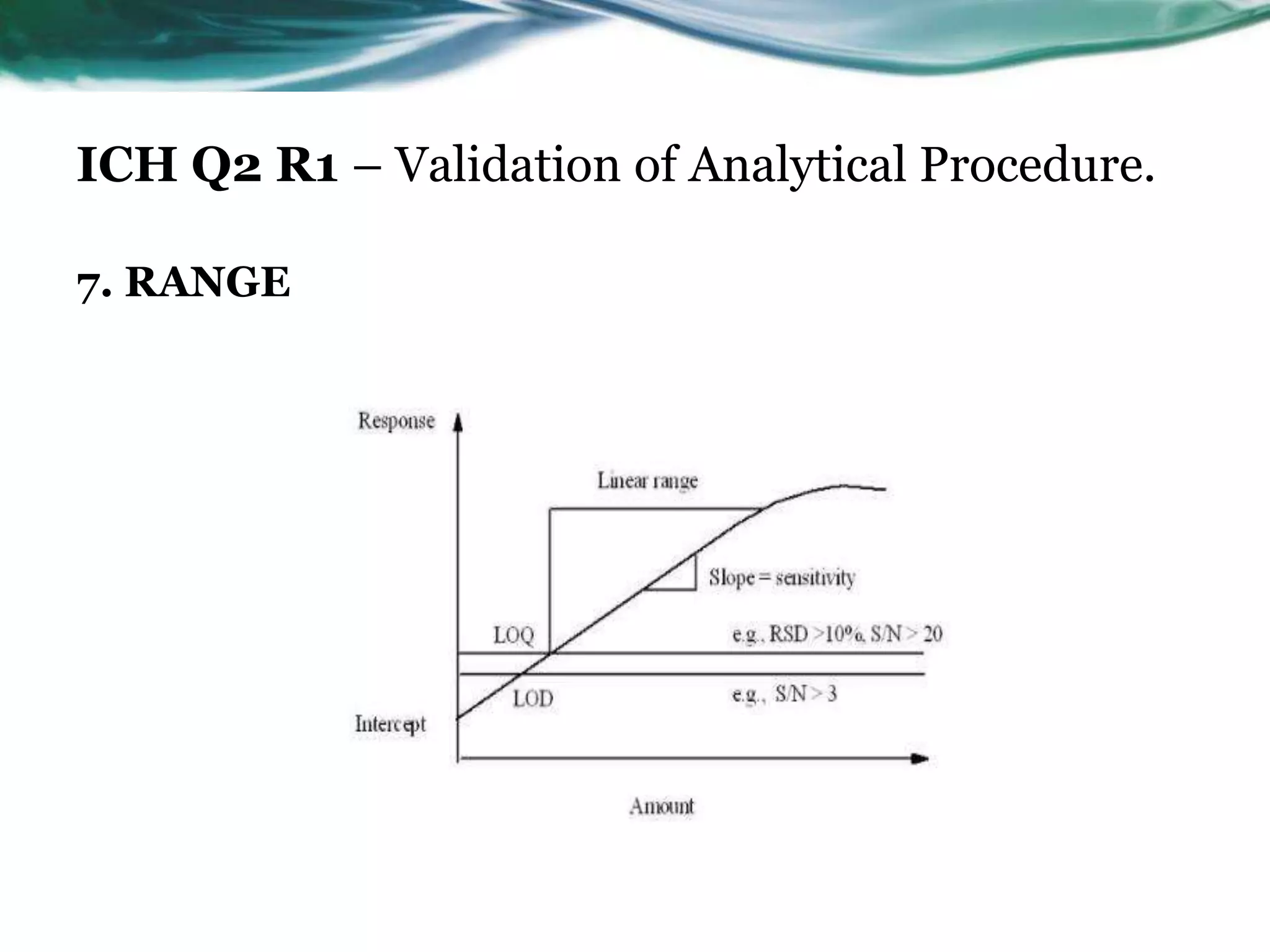
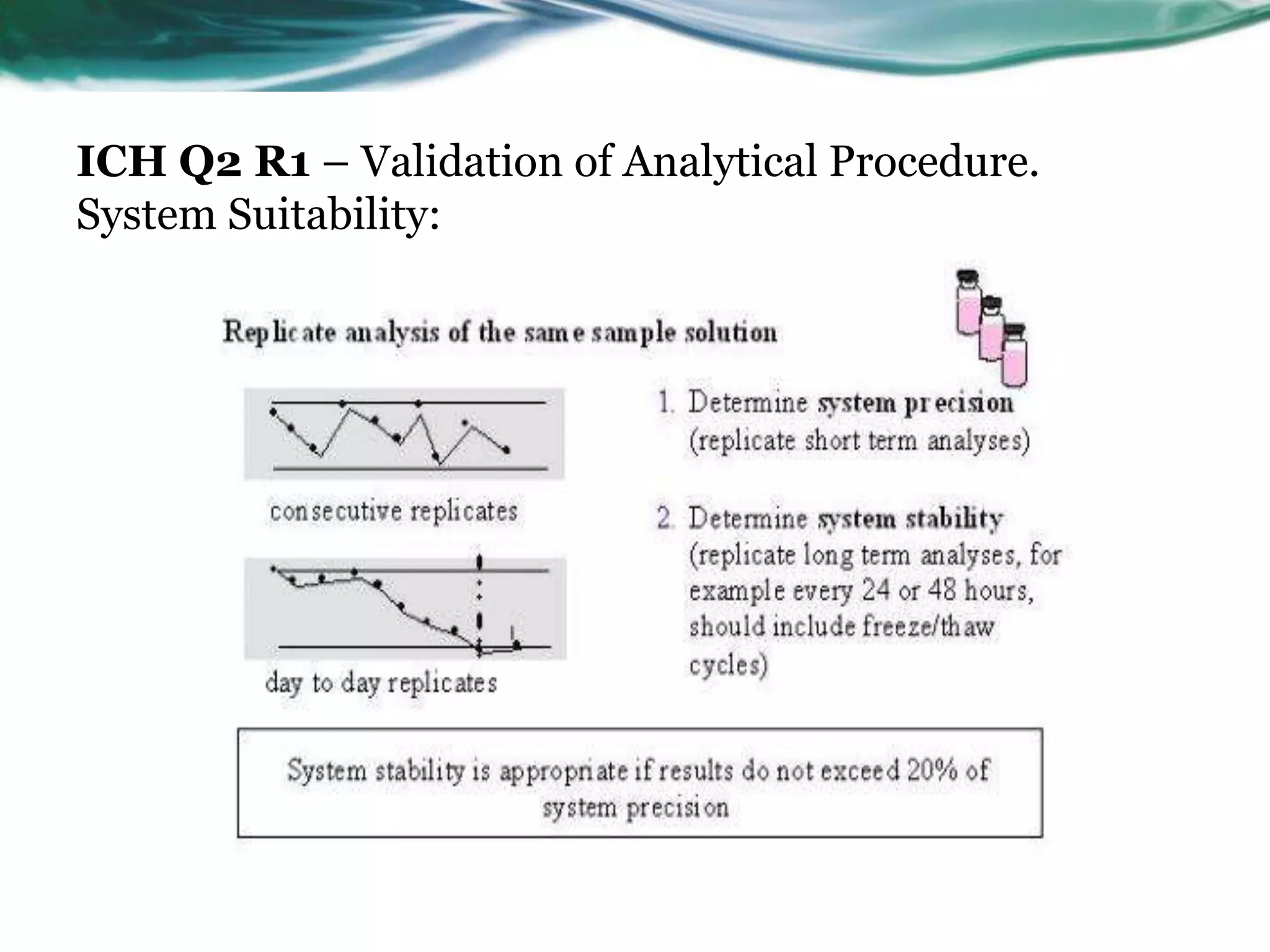
This document summarizes the ICH Q2 R1 Guideline on validation of analytical procedures. It discusses the objective of validation, which is to demonstrate that an analytical procedure is suitable for its intended purpose. It outlines the types of analytical procedures that should be validated, including identification tests, quantitative impurity tests, limit tests for impurities, and assay procedures. It also describes the key validation characteristics that should be tested, such as specificity, accuracy, precision, detection limit, quantitation limit, linearity, range, robustness, and system suitability. The document provides details on these validation parameters and recommends the type of data that should be collected for each parameter. It also discusses related topics like method verification versus validation and re

















![VERIFICATION
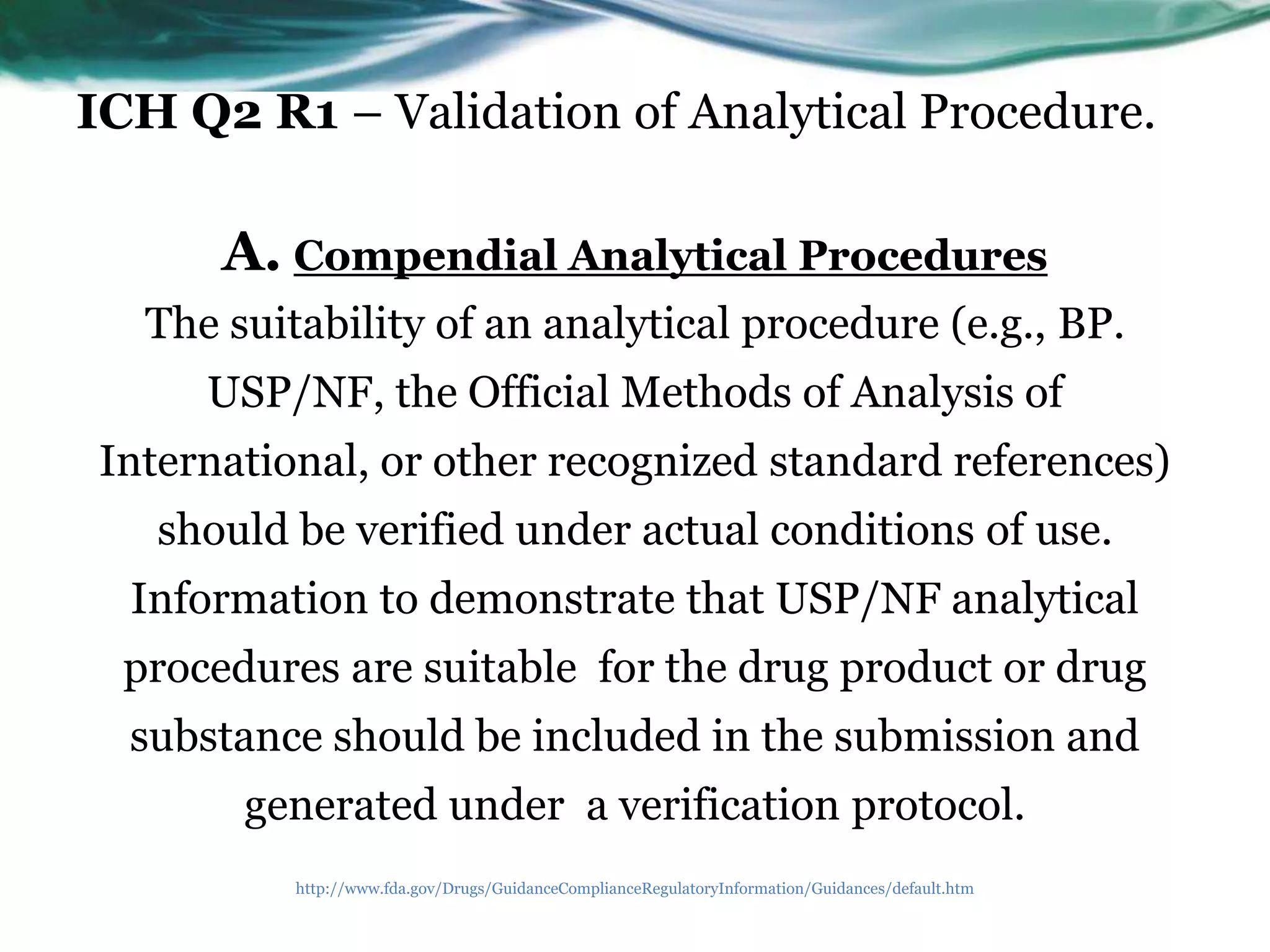
USP <1225> states:
†
“According to these regulations [21 CFR
211.194(a)(2)], users of analytical methods described
in USP-NF are not required to validate the accuracy
and reliability of these methods, but merely verify their
suitability under actual conditions of use.”
…
USP <1226> states:
†
“Users of compendial analytical procedures are not
required to validate these procedures when first used
in their laboratories, but documented evidence of
suitability should be established under actual
conditions of use.”](https://image.slidesharecdn.com/q2methodvalidation-161105173750-210609202434/75/Q2methodvalidation-161105173750-47-2048.jpg)


