This document outlines the key steps in method validation for clinical chemistry laboratories. It defines method validation and discusses when methods should be validated. The key aspects that must be validated include calibration, precision, accuracy, linearity, limit of detection, analytical range, sensitivity, specificity, ruggedness and robustness. Thorough documentation of the validation study is also required. Validating a method involves experimentally testing these parameters and documenting the results to provide objective evidence that the method is suitable for its intended use.










![I. CALIBRATION
o Established by measurement of samples with known quantities of the analyte
(calibrators). [Only certified reference materials with known traceability should
be used]
o Done by- Chemical standards or samples with known quantities of analyte
present in the typical matrix.
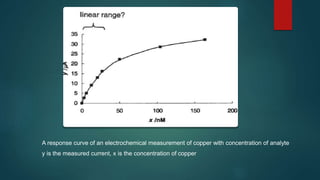
o Calibration functions- may be linear or curved.
o If the assumed calibration function does not correctly reflect the true
relationship between instrument response & analyte concentration, a systemic
error or bias is likely to be associated with the analytical method.](https://image.slidesharecdn.com/methodvalidation-140901112023-phpapp02/85/Method-validation-11-320.jpg)