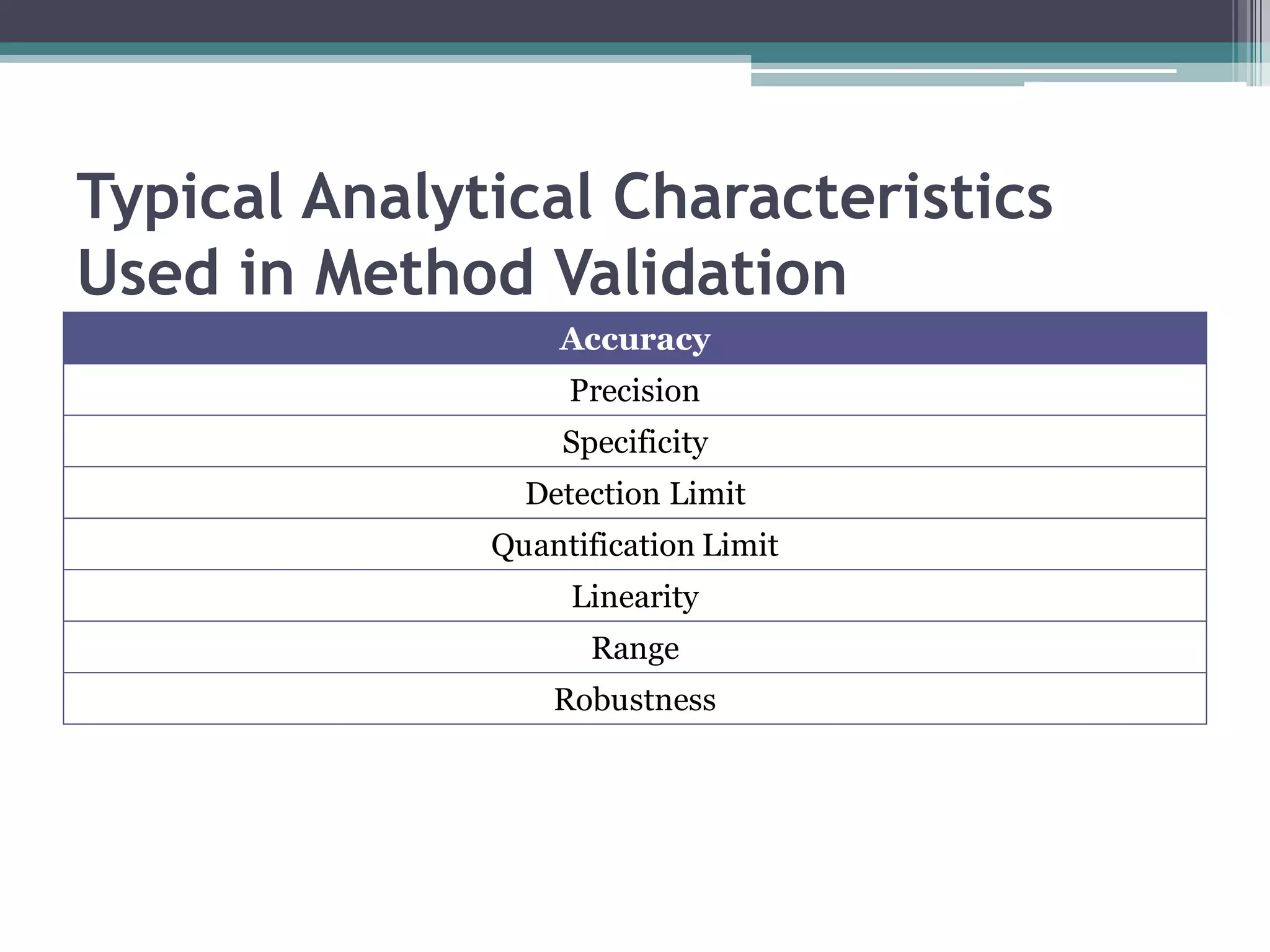
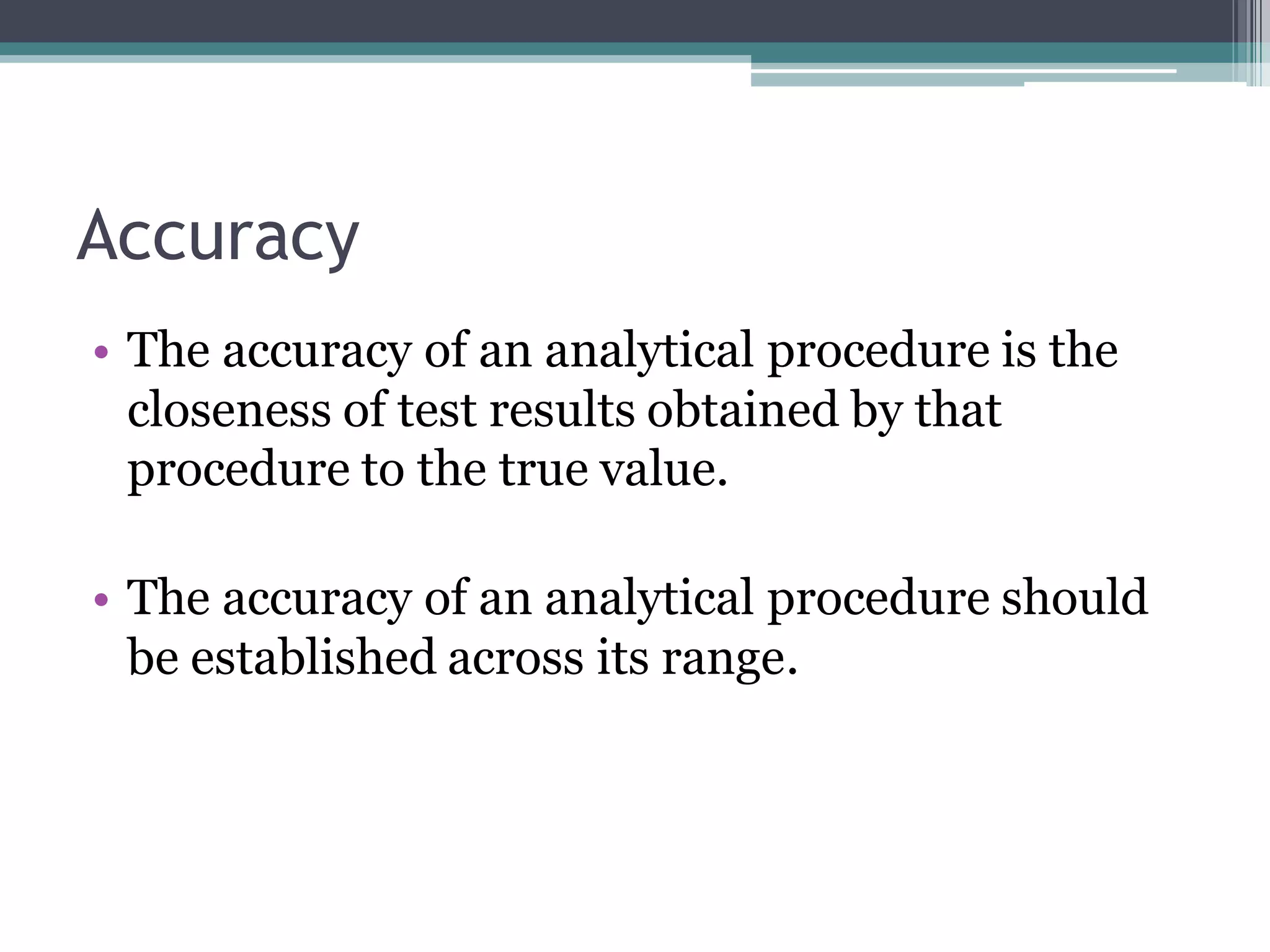
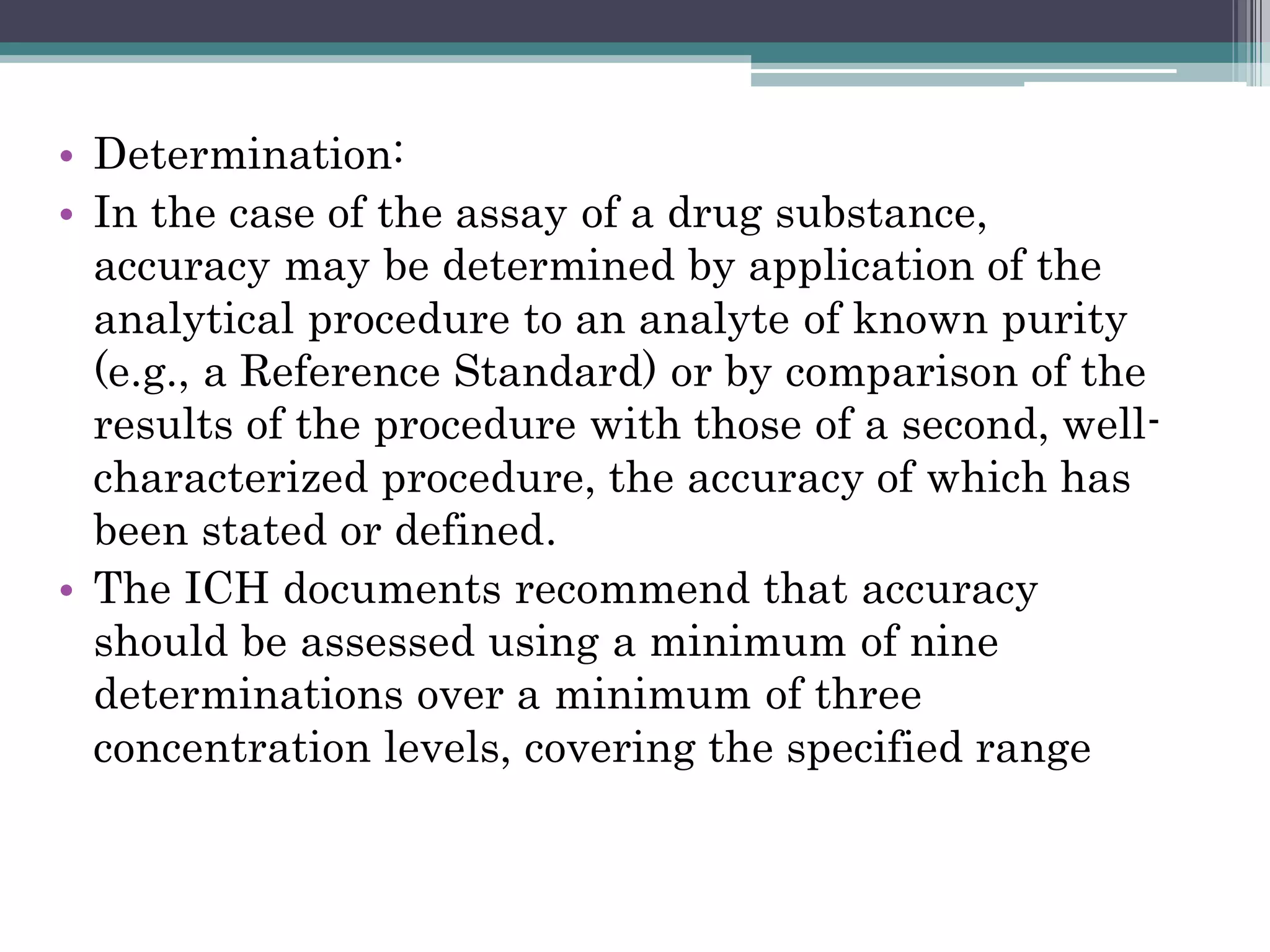
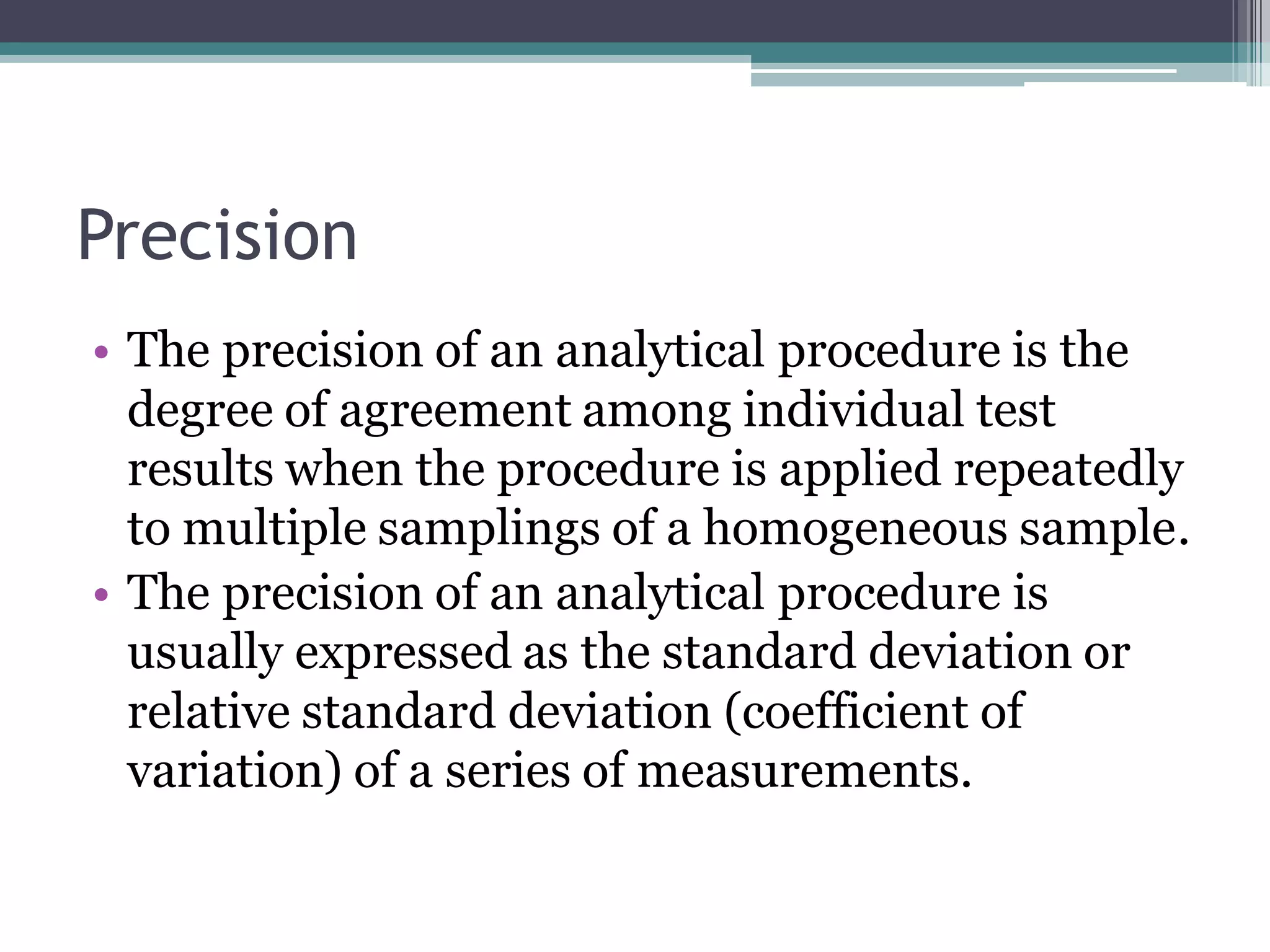
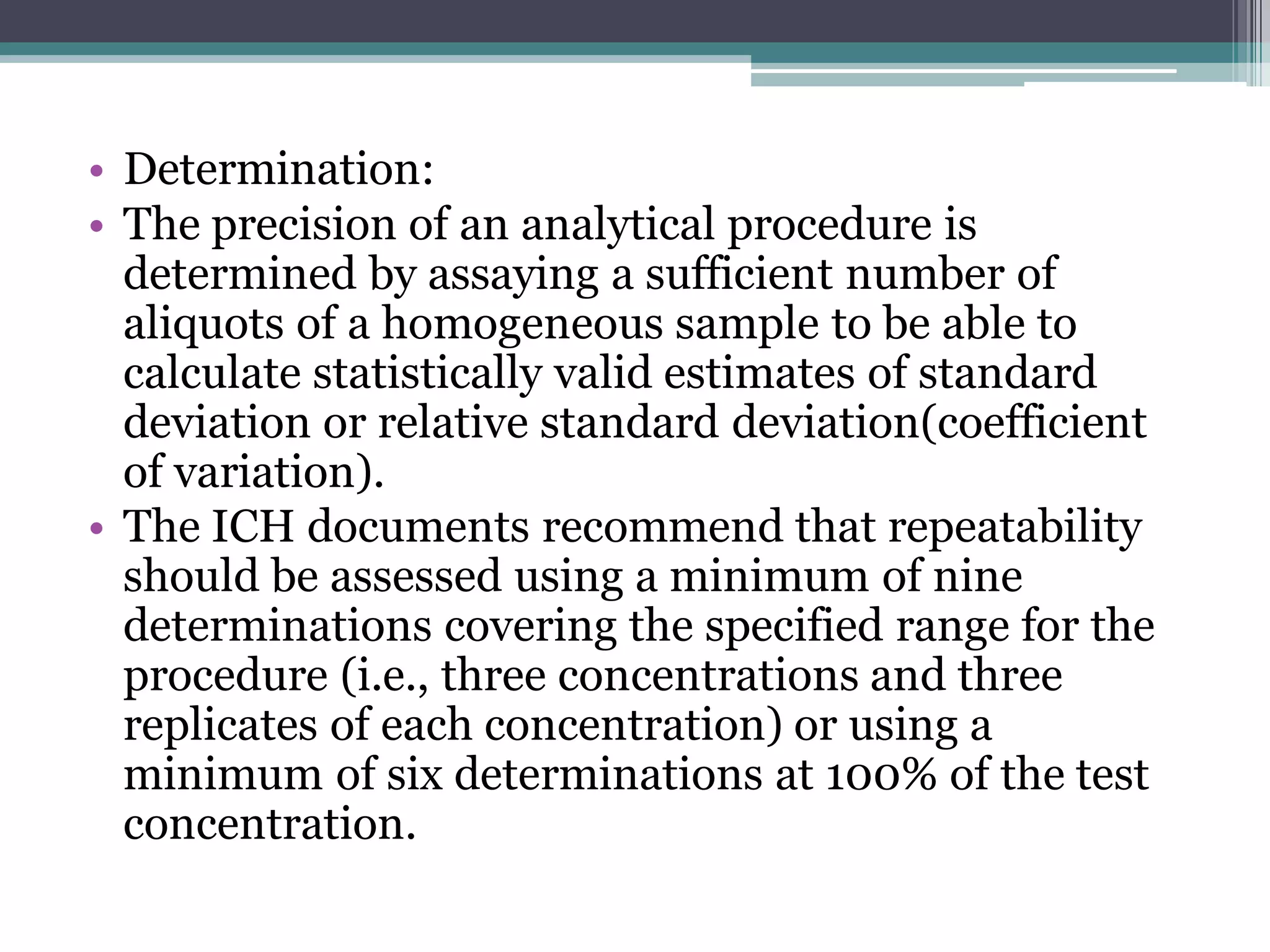
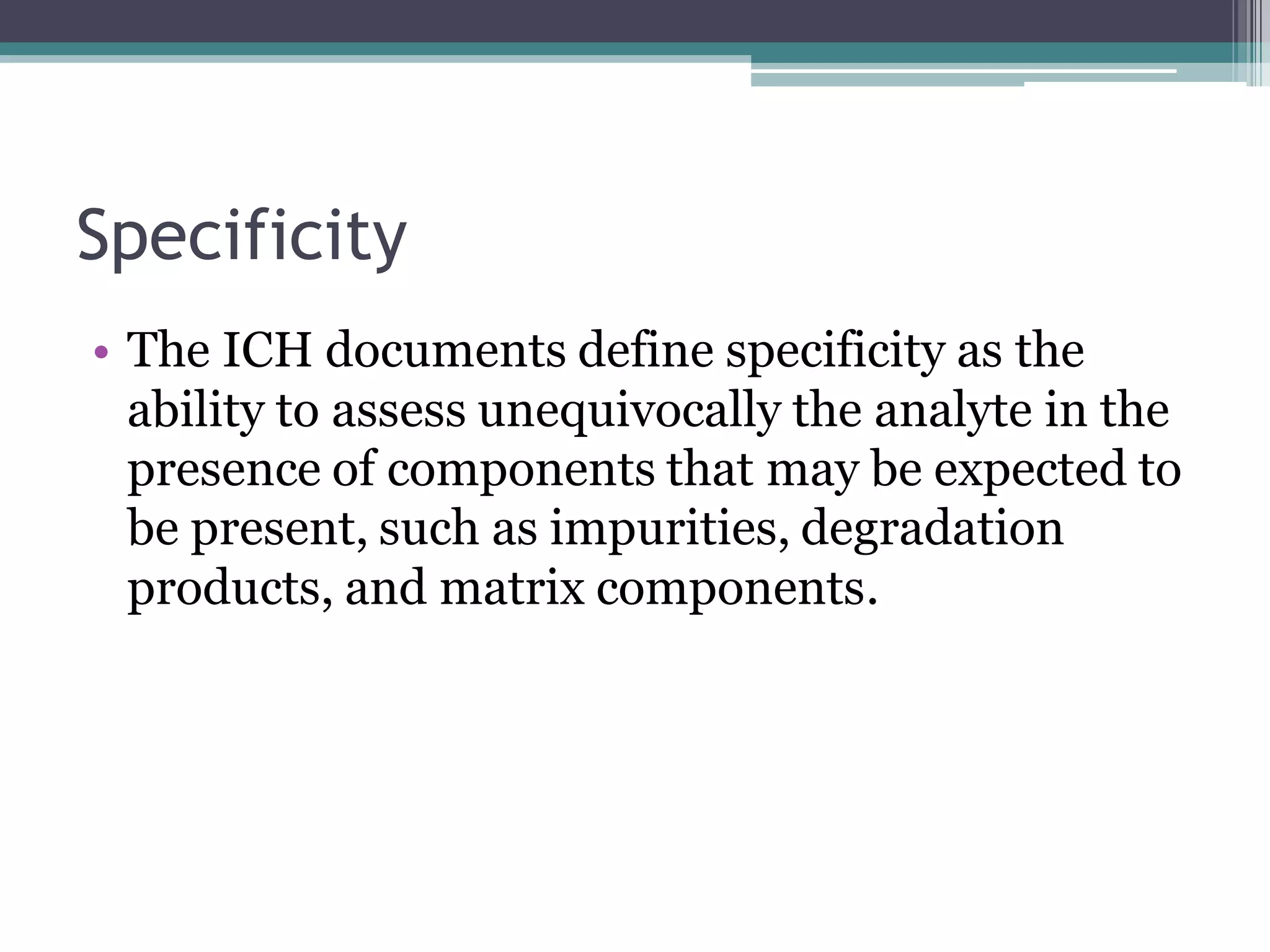
The document discusses the validation of analytical procedures as outlined by regulatory agencies like the USFDA. It defines key terms like accuracy, precision, specificity, detection limit, quantitation limit, linearity, range, and robustness. For each term, it provides the definition and recommendations on how to determine the characteristic during the validation process, such as testing a minimum number of samples over a specified range and concentration levels. The overall purpose of the validation is to establish that the analytical procedure is suitable for its intended use.