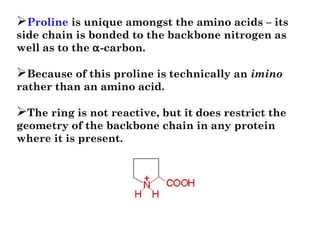
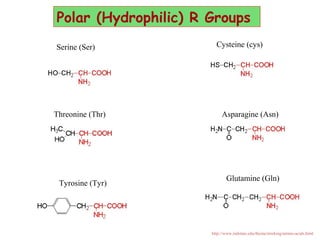
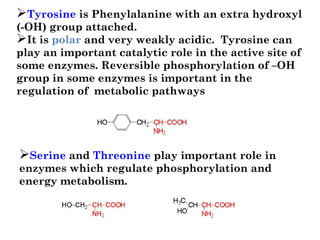
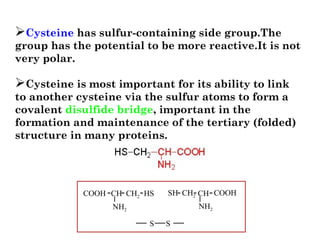
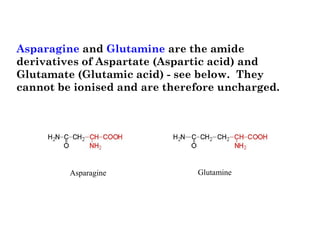
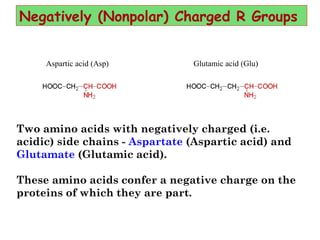
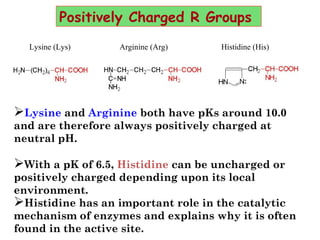
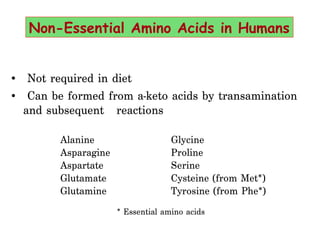
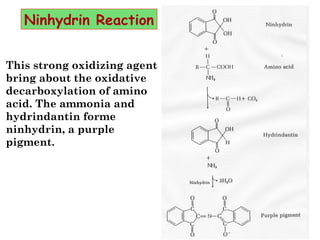
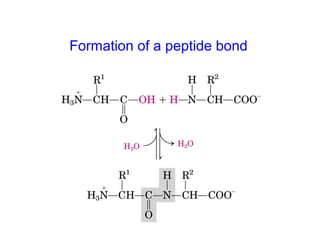
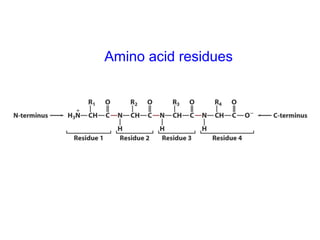
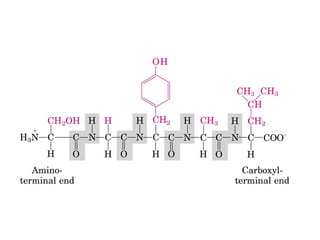
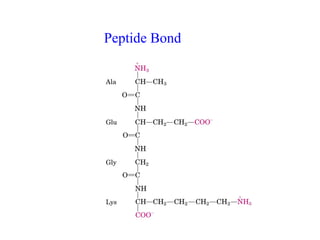
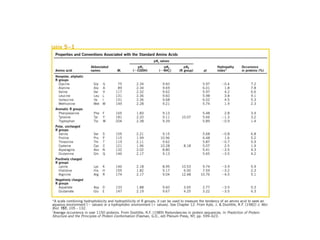
- Amino acids are the building blocks of proteins and there are 20 naturally occurring amino acids.
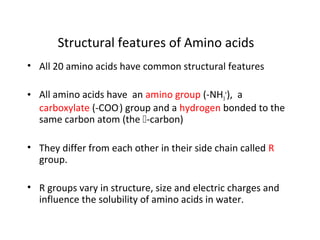
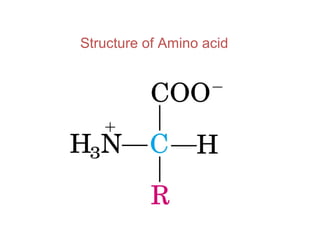
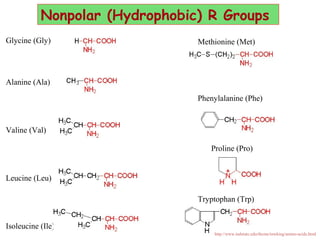
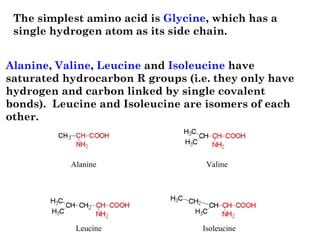
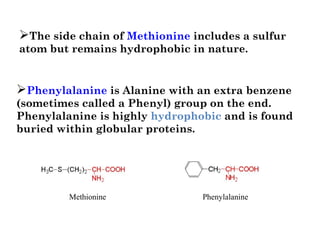
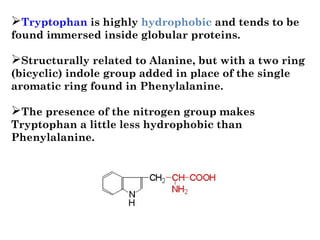
- Amino acids have common structural features including an amino group, carboxyl group, and R group that differs between each amino acid. The R group influences solubility.
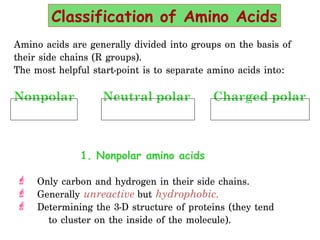
- Amino acids can be classified as nonpolar, polar, negatively charged, or positively charged based on their R groups. This classification impacts properties like hydrophobicity.
- Ten amino acids are considered essential in humans as we cannot synthesize them and must obtain them from diet. The other ten are non-essential as our bodies can produce them.