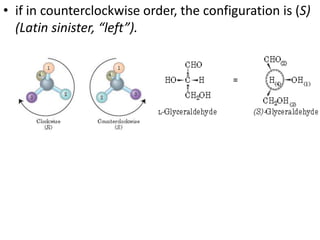
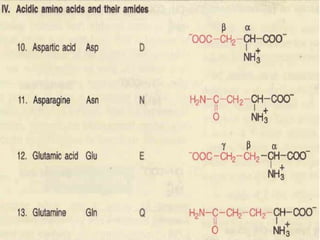
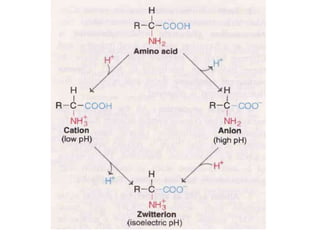
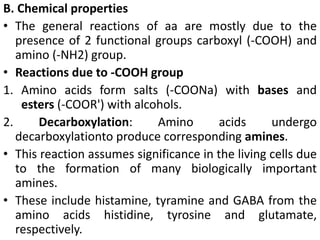
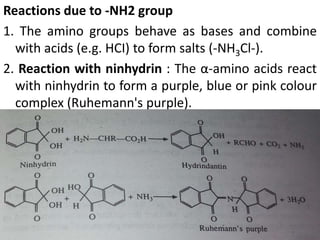
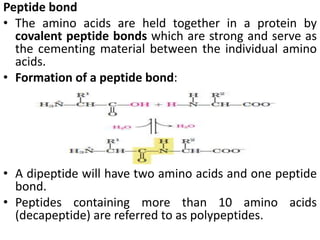
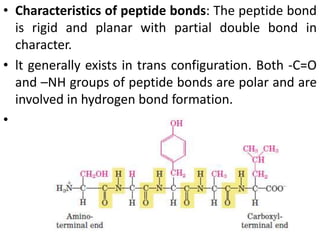
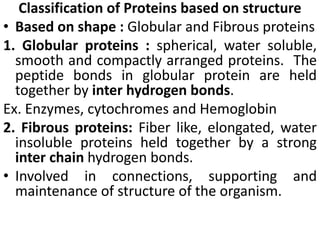
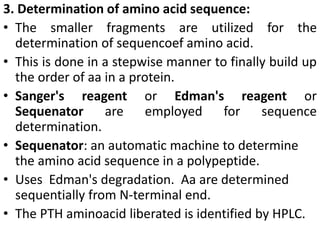
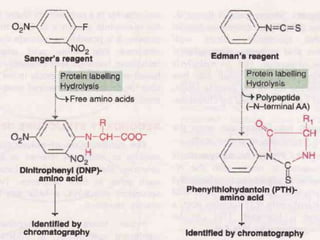
Proteins are polymers of amino acids and perform a variety of essential functions in living cells. They can be classified based on their structure, composition, and properties. The main types are globular and fibrous proteins. Globular proteins are spherical and soluble, while fibrous proteins are elongated and form connections between tissues. Proteins are also classified as simple proteins containing only amino acids, or conjugated proteins which contain non-amino acid groups like carbohydrates, lipids, or metals. Amino acids polymerize to form peptide bonds, linking them into protein chains.