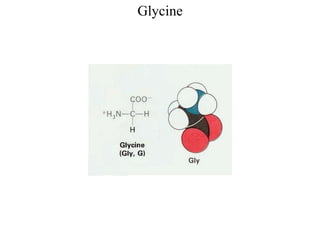
This document discusses the structure and properties of amino acids and proteins. It begins by defining amino acids as the building blocks of proteins and discusses their various classifications including essential vs non-essential amino acids, polarity, and metabolic fate. It then covers the physical properties of amino acids such as solubility, melting point, and their amphoteric nature. Finally, it discusses protein structure, describing the primary, secondary, tertiary and quaternary levels as well as different types of proteins classified by their function.


![Classification of Amino Acids
A.Classification based on structure:
Each amino acid is assigned a 3 letter or 1 letter
symbol
These symbols are commonly used to represent the
amino acids in protein structure
e.g.:
ANGIOTENSIN II (octapeptide)
[Asp-Arg-Val-Tyr-Ile-His-Pro-Phe]
or simply
[D-R-V-Y-I-H-P-F]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-6-320.jpg)

![Classification of amino acids based on polarity
There are four groups of AA depending topolarity
1.Non-polar AA (also referred as hydrophobic [water hating]) nocharge
on ‘R’group
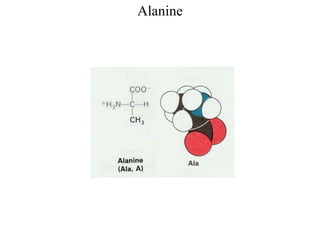
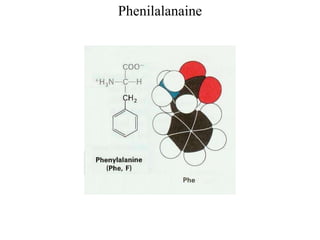
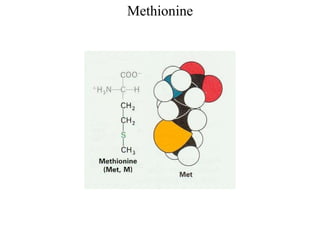
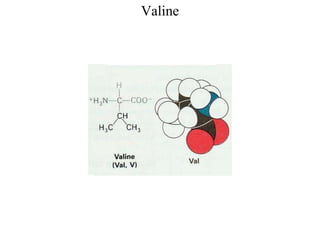
[Alanine, Leucine, Isoleucine, Valine, Methionine, Phenylalanine,
Tryptophan, Proline]
2.PolarAAwith no charge on ‘R’group
[Glycine, Serine, Threonine, Cysteine, Glutamine, Asparagine.Tyrosine]
– they however possess groups such as hydroxyl, sulfhydryl, amide –
and participate in hydrogen bonding of protein structure.
3.PolarAAwith positive ‘R’group
[Lysine, Arginine, Histidine]
4.PolarAAwith negative ‘R’group
[Aspartic acid, GlutamicAcid]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-20-320.jpg)

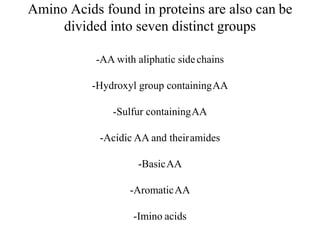
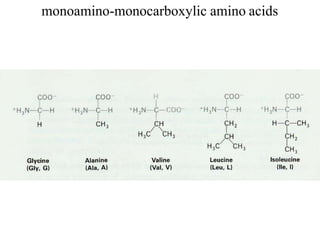
![Aliphatic amino acids
[Glycin, Alanin, Valin, Leucin, Isoleucin]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-28-320.jpg)
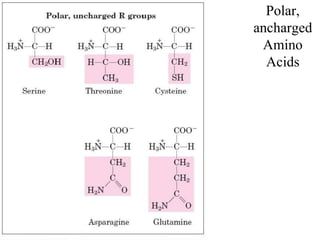
![Hydroxyl group containing AA
[Serine, Threonine, Tyrosine]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-29-320.jpg)
![Sulfur containing AA
[Cystein (with sulfhydryl group), Methionine (with thioether group)]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-30-320.jpg)
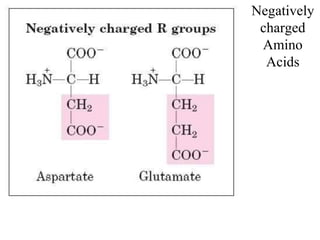
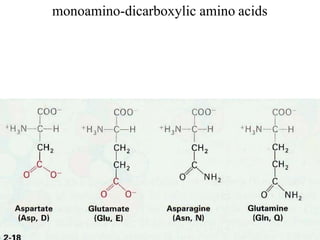
![Acidic Amino acids and their amides
[Aspertic acid (Aspartate), Glutamic acid (Glutamate)] – dicarboxylic
monoamino acids
[Aspargine, Glutamine] – their respective amide derivatives
all these fourAApossess distinct codons for their incorporation into
proteins](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-31-320.jpg)
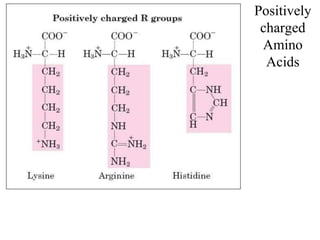
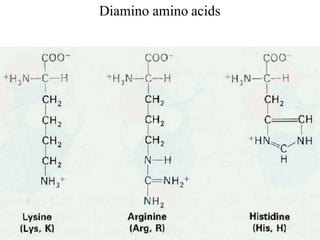
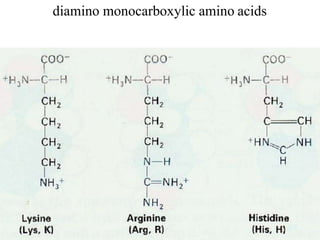
![Basic AA
[Lysine, Arginine (with gunidino
group), histidine (imidazole ring)]
dibasic monocarboxylic acids (they
are highly basic in character)](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-32-320.jpg)
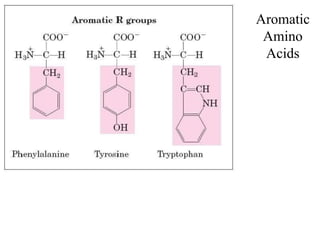
![Aromatic AA
[Phenilalanine, Tyrosine, Tryptophan (with indolering)]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-33-320.jpg)
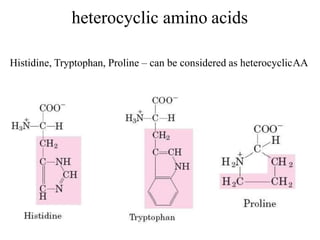
![Imino acids
[Proline (containing pyrrolidine ring)] – it has an imino group (=NH)
instead of amino group (–NH2). Therefore Proline is α-imino acid](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-34-320.jpg)



![AA classification based on their metabolic fate
Carbone skeleton of AA can serve as a precursor for the synthesisof
glucose (glycogenic) or fat (ketogenic) or both.
1.Glycogenic AA (can serve as precursors for the formation of glucoseor
glycogen)
[Alanine, Aspartate, Glycin, Methionineetc]
2.Ketonic AA (fat can besynthesized)
[Leucine, Lysine]
3.Glycogenic and kethogenicAA
[Isoleucine, Phenylalanine, Tryptophan, Tyrosine] – precursors for
synthesis of glucose as well as fat.](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-44-320.jpg)


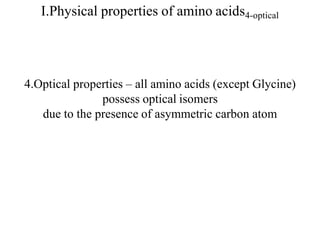
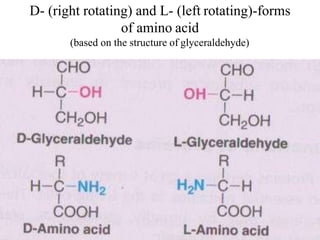
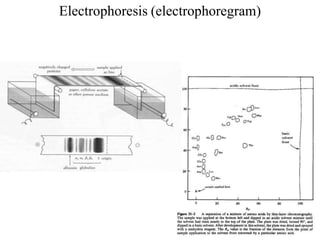
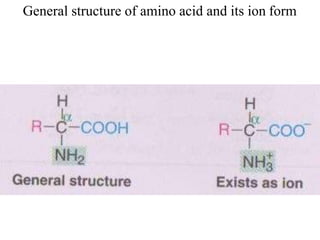
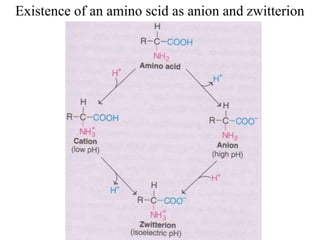
![I.Physical properties of amino acids5-ampholytes
Amiono acids as ampholytes
AA contain both acidic (–COOH) and basic (–NH2) groups.
They can donate a proton or accept a proton – hence AAare regarded as
ampholytes.
Zwitter ion (or dipolar ion) – [name zwitter is from German – means
hybrid].
Is a hybrid molecule containing positive and negative ionic groups.
The AA rarely exist in a neutral form with free carboxylic(–COOH) and
free amino [basic] (–NH2) groups. In strongly acidic pH (low pH), the
amino acid is positively charged (cation), while in strongly alkaline pH
(high pH), it is negatively charged (anion). Each AA has acharacteristic
pH at which it carries positive and negative charges and exists as
zwitterions.](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-50-320.jpg)
![Titration curve of amino acid [Leucine]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-54-320.jpg)

![II2. Chemical properties of amino acids
Amino acids undergo decarboxylation – to produce
corresponding amines
Reactions due to –COOH group
this reaction assumes significance in the living cells due to the
formation of many biologically important amines [histamine
(from histidine), tyramine (from tyrosine), γ-amino buteric
acid (GABA) – from grutamate]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-56-320.jpg)




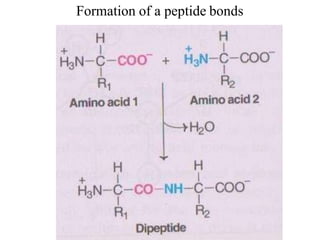
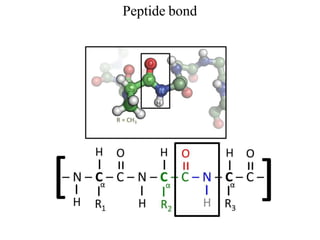
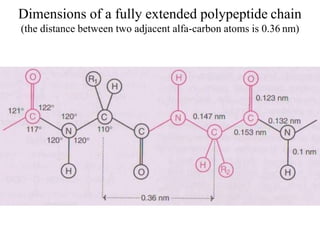
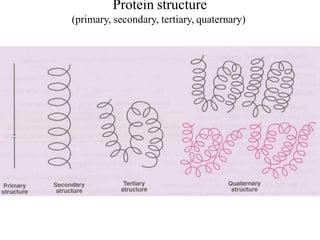
![Secondary structure of protein
A right handed alfa-helix
–CH–R groups of amino acids;
Dotted blue lines are hydrogen
bonds.
[here only a few hydrogen bonds shown
for clarity]](https://image.slidesharecdn.com/1-170703091850/85/Amino-Acids-Peptides-Proteins-70-320.jpg)