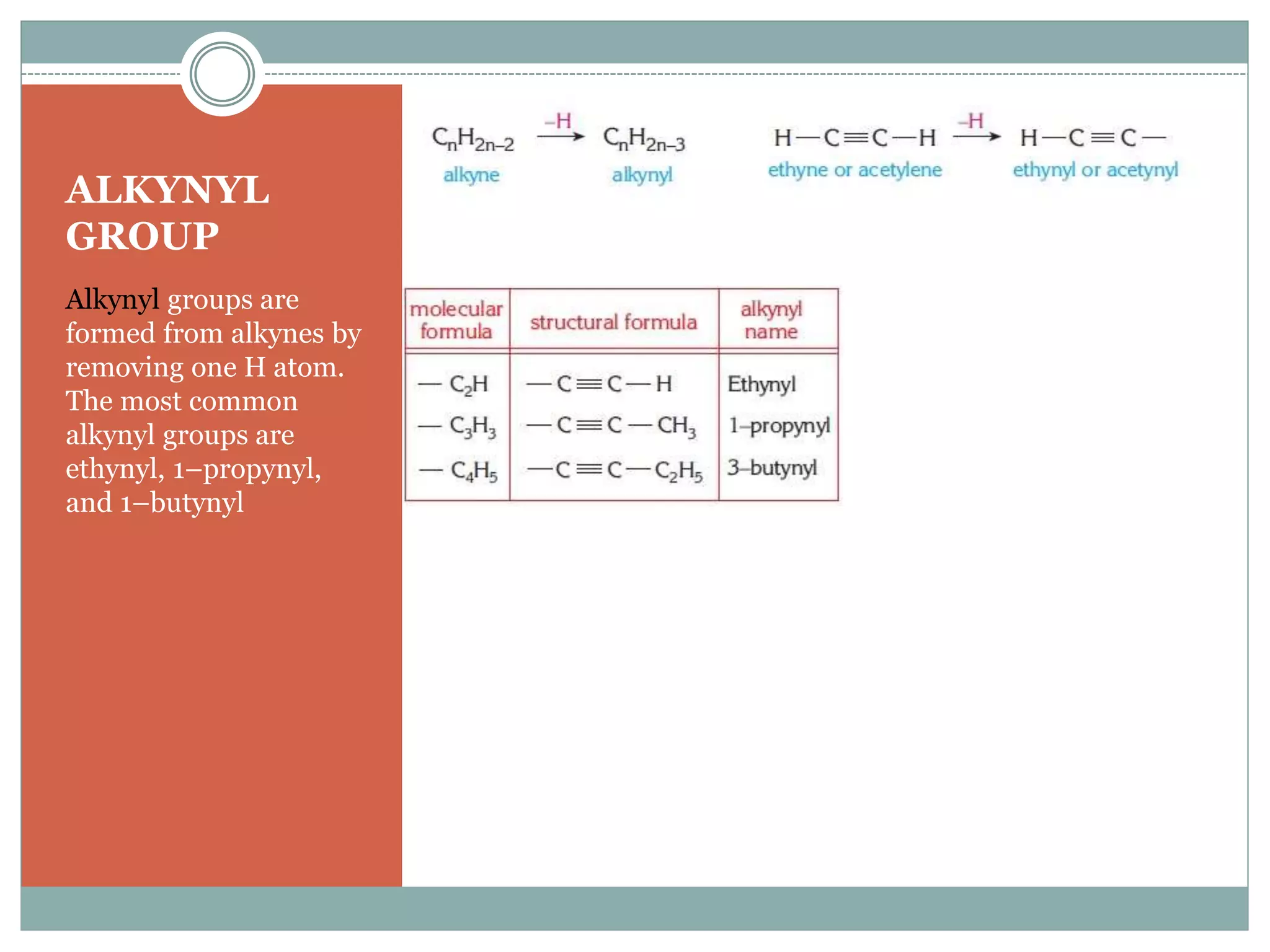
1) Alkynes are hydrocarbons containing a carbon-carbon triple bond. Their general formula is CnH2n-2.
2) Alkynes undergo combustion reactions and addition reactions like alkenes. They can also undergo substitution reactions with metals.
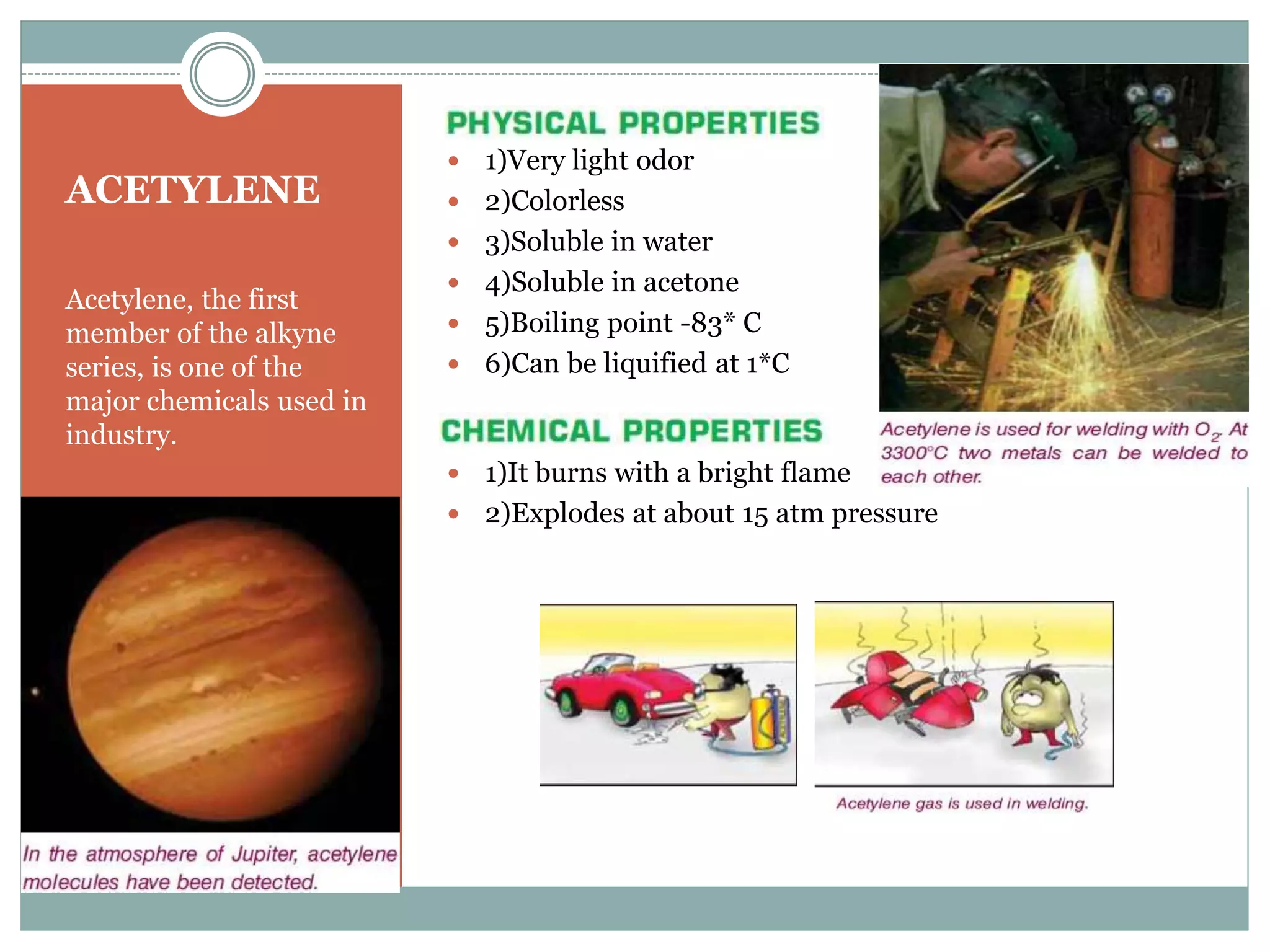
3) Important alkynes include acetylene, which is soluble in water and organic solvents and burns with a bright flame. It is used widely in industry.