This chapter discusses alkynes, also known as acetylenes, which are unsaturated hydrocarbons containing carbon-carbon triple bonds. The key points covered include:
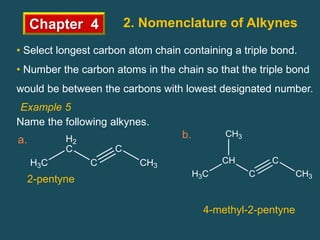
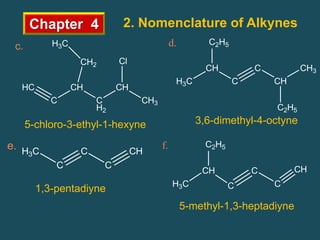
- Alkynes follow the general formula CnH2n-2 and have sp hybridized carbons in the triple bond. Nomenclature involves changing the -ane ending to -yne.
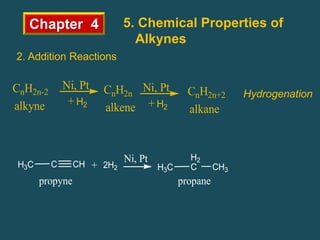
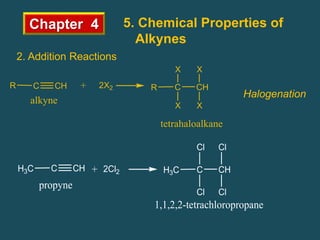
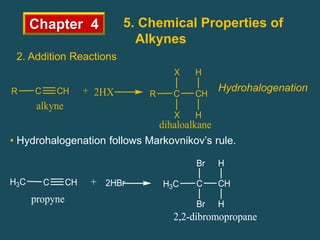
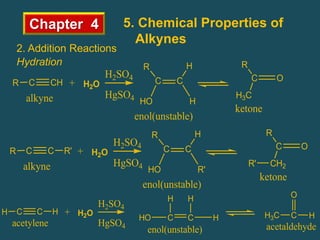
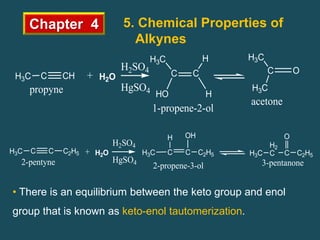
- Alkynes can undergo addition reactions like hydrogenation, halogenation, and hydration. Hydration produces ketones or aldehydes depending on the alkyne.
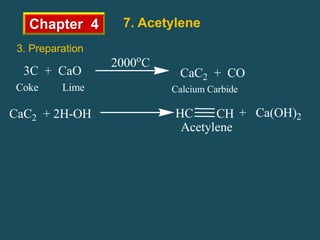
- Acetylene is the simplest and most common alkyne. It is a colorless, flammable gas that is produced commercially