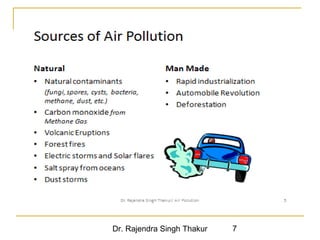
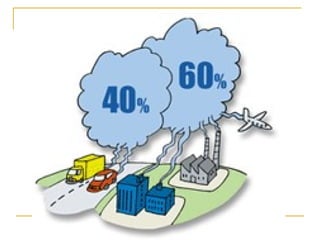
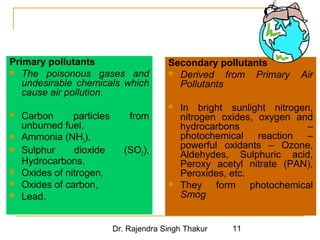
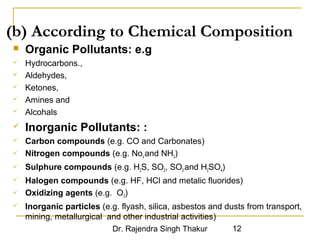
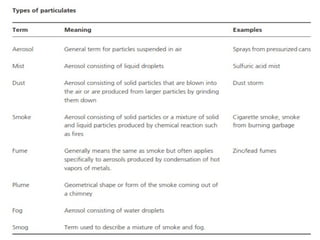
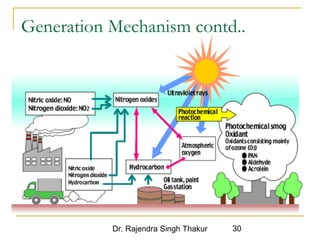
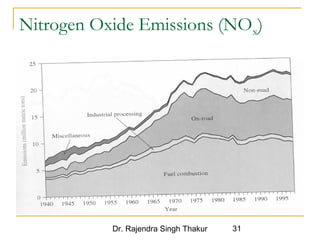
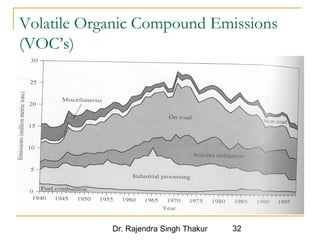
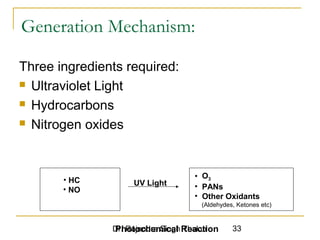
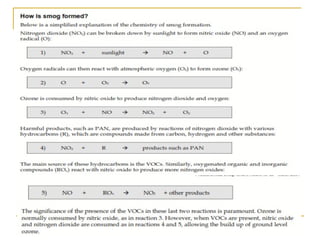
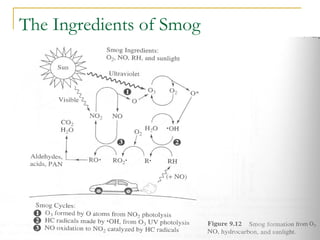
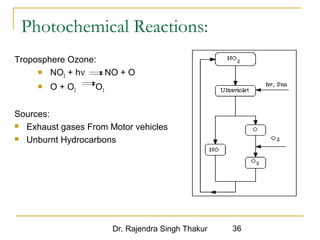
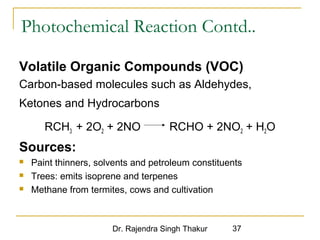
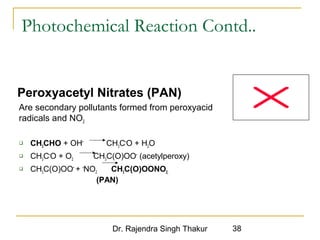
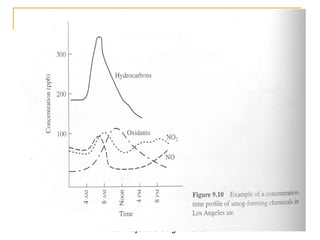
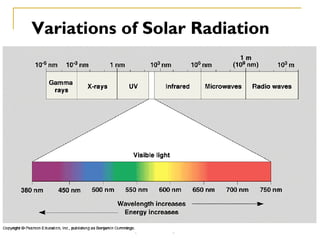
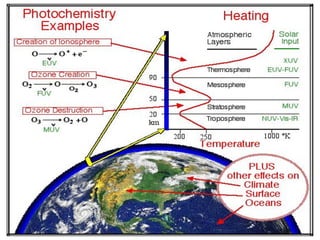
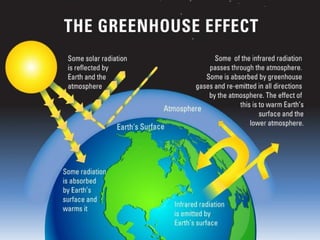
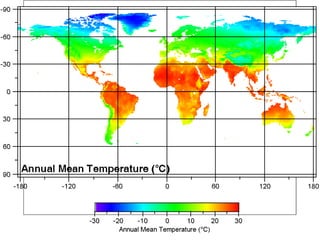
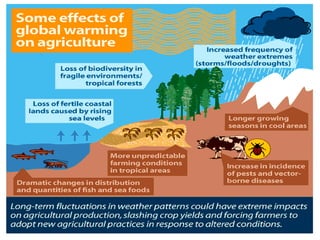
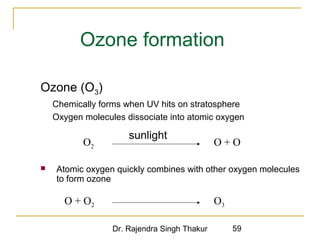
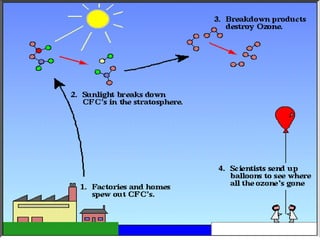
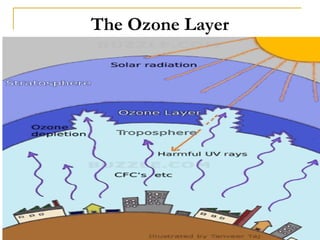
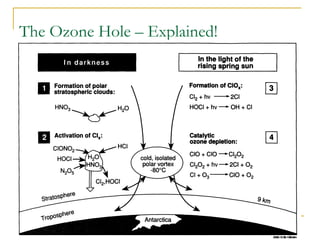
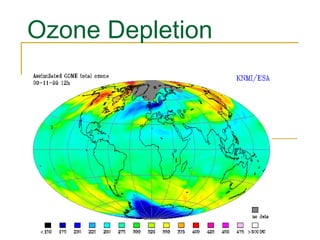
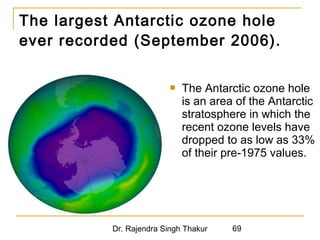
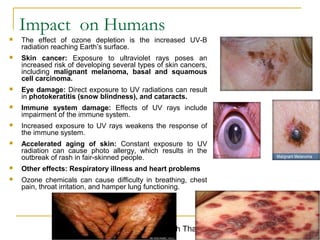
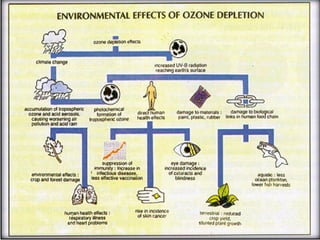
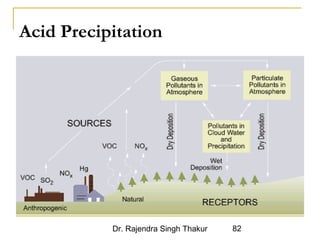
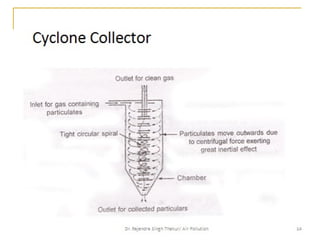
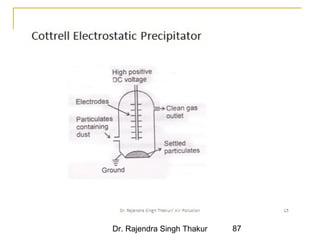
The document provides a comprehensive overview of air pollution, defining it as the presence of harmful chemicals in the atmosphere due to human activities like burning fossil fuels and industrial processes. It categorizes air pollutants into primary and secondary based on their origin and explains their effects on human health, plants, animals, and the environment, including the formation of photochemical smog and the greenhouse effect. Various sources and types of pollutants are discussed, highlighting their biochemical impacts and the need for awareness of transboundary pollution issues.