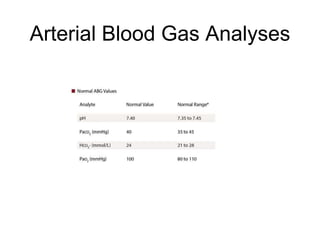
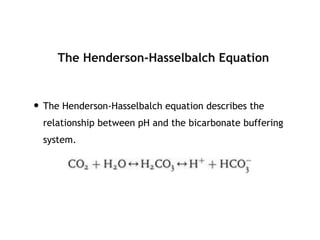
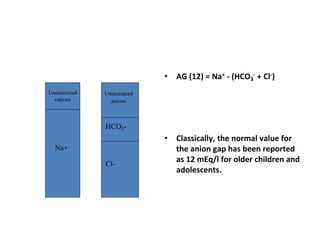
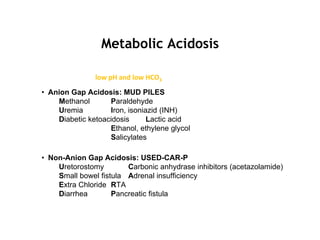
This document discusses acid-base disorders. It begins by outlining the aims of examining pH, determining the primary disorder, calculating the anion gap, assessing compensation, and defining the disorder and treatment. It then provides introductions to pH, the Henderson-Hasselbalch equation, arterial blood gas analyses, and the anion gap. The document proceeds to discuss types of acid-base disorders including metabolic acidosis, respiratory acidosis, metabolic alkalosis, and respiratory alkalosis. It covers causes, symptoms, and treatments for each. An example case is presented of a patient with respiratory alkalosis.




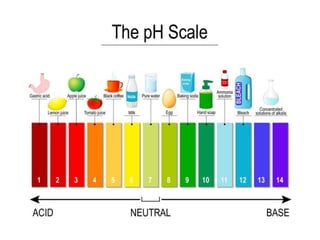
![What is pH?
•The precise definition of pH is…
pH = - log 10 ([H+])](https://image.slidesharecdn.com/acid-basedisorderyusufcingirlar-190327185447/85/Acid-base-disorder-5-320.jpg)