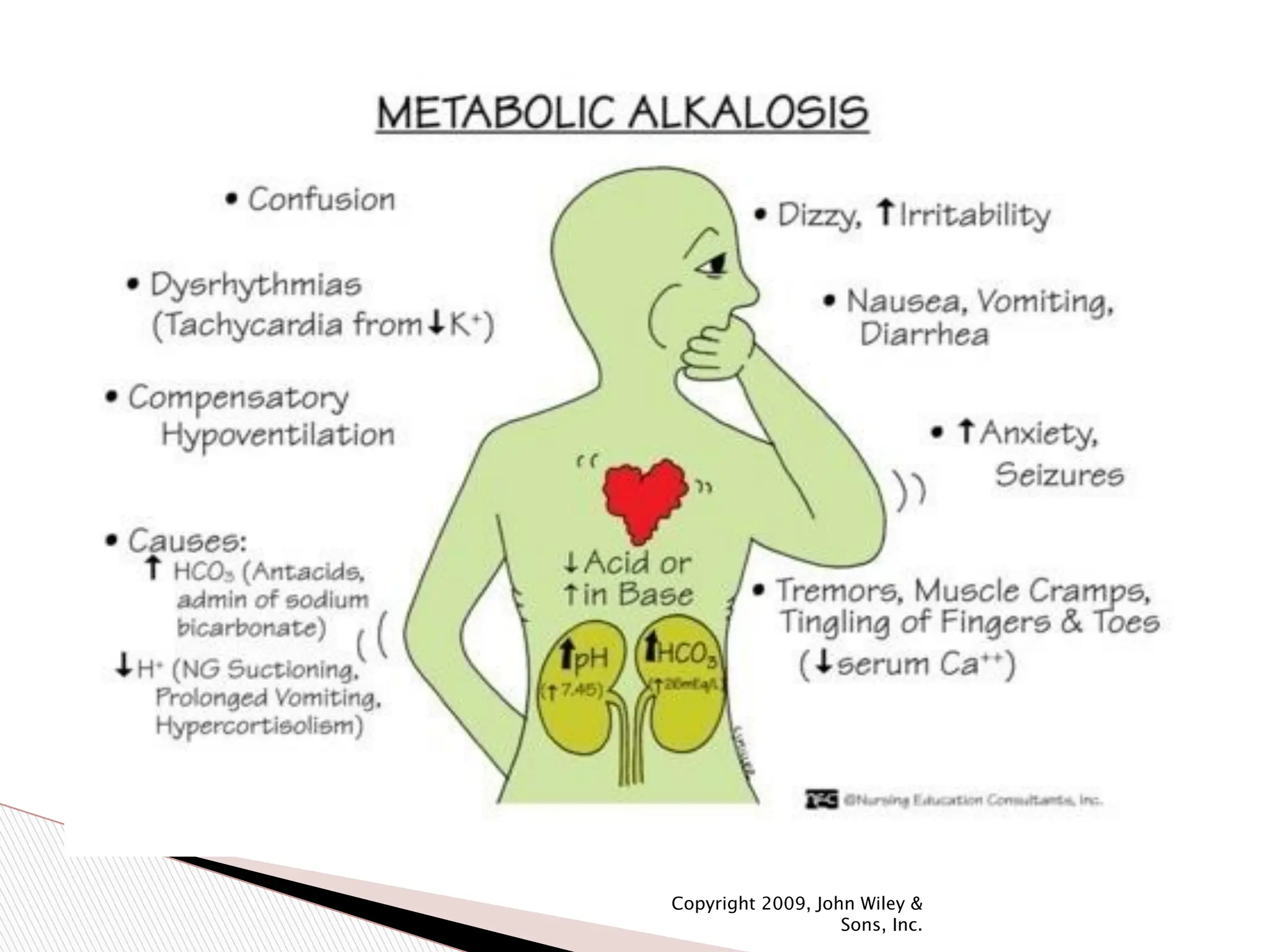
The document provides an in-depth overview of acid-base imbalances, detailing acidosis and alkalosis, their physiological effects, and compensation mechanisms related to both metabolic and respiratory disturbances. It includes diagnostic information, the role of buffers, and specific medical conditions leading to imbalances, along with treatment approaches. Additionally, it covers concepts such as the anion gap and the 'alkaline tide' phenomena associated with food intake.





![pH = pKₐ + log([A⁻]/[HA])
[A-
] = conjugate base or salt
[HA] = acid
Henderson–Hasselbalch equation](https://image.slidesharecdn.com/acidbaseimbalance-2-241225155205-5836fb4e/75/Acid-base-imbalance-compensations-causes-6-2048.jpg)

































![Anion gap : difference between unmeasured anions and
unmeasured cations (only a diagnostic concept)
Plasma anion gap = [Na+
] - [HCO3
-
] - [Cl-
]
= 144 - 24 - 108
= 12 mEq/L
Concentration units : mEq/L or in mmol/L
Anion gap increase if unmeasured anions rise or if unmeasured
cations fall.
Major unmeasured cations :Ca++
,Mg+2
,K+
, gamma globulins
Major unmeasured anions : albumin , phosphate, sulfate, lactate
& other organic anions.
Anion gap range : 8 -16 mEq/L as unmeasured anions exceed
unmeasured cations
Anion gap](https://image.slidesharecdn.com/acidbaseimbalance-2-241225155205-5836fb4e/75/Acid-base-imbalance-compensations-causes-41-2048.jpg)
![Calculation of anion gap: calculated by subtracting the serum
concentrations of chloride and bicarbonate (anions) from the
concentrations of sodium and potassium (cations):
= ( [Na+
]+[K+
] ) − ( [Cl−
]+[HCO3
−
] )
Without potassium (daily practice)
Because potassium concentrations are very low, they usually
have little effect on the calculated gap. omission of potassium
has become widely accepted. This leaves the following equation:
= ( [Na+
] ) − ( [Cl−
]+[HCO3
−
] )
average anion gap for healthy adults : 8-12 mEq/L](https://image.slidesharecdn.com/acidbaseimbalance-2-241225155205-5836fb4e/75/Acid-base-imbalance-compensations-causes-42-2048.jpg)