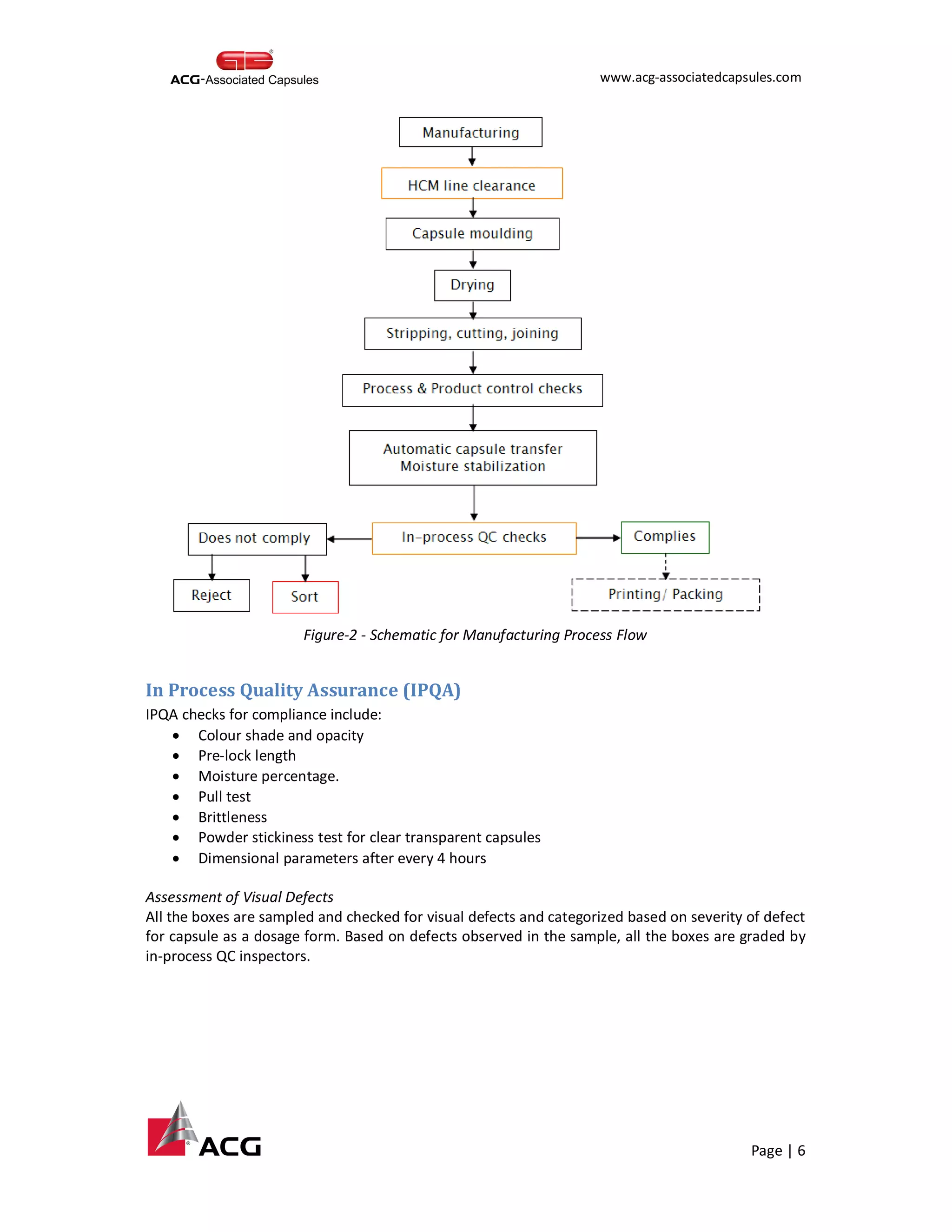
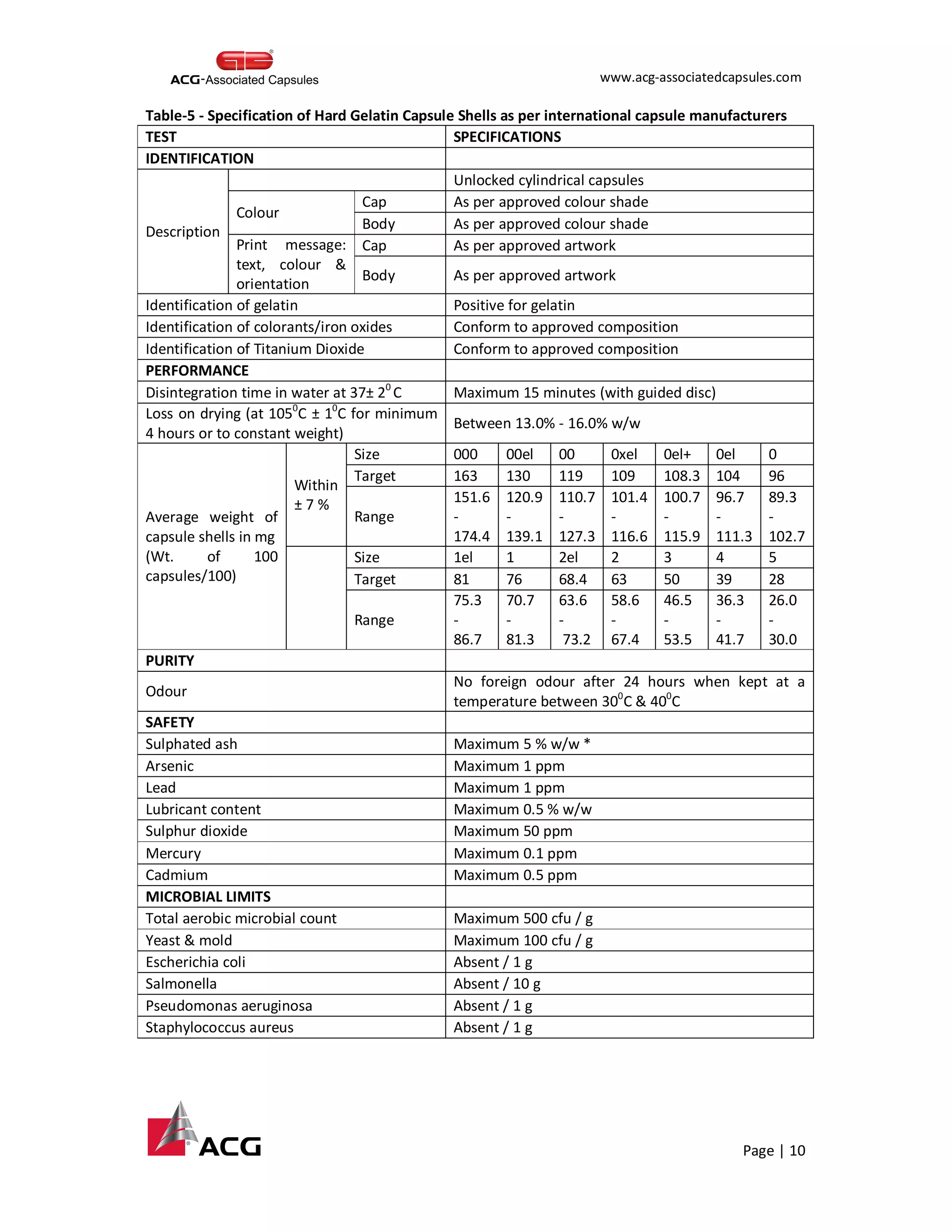
This document provides an overview of the process for manufacturing hard gelatin capsules. It discusses the key raw materials used, including bovine gelatin and food colors. The manufacturing process involves preparing a gelatin mucilage solution, dipping pin bars in the solution to form capsule shells, drying the shells, cutting and joining them, and performing quality checks. The capsules then undergo printing and packaging, with quality testing of raw materials, in-process materials, and finished products. Specifications are provided for testing various attributes of the hard gelatin capsule shells.